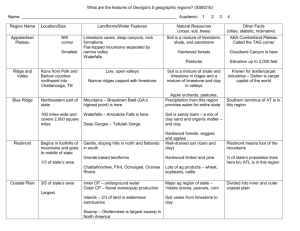
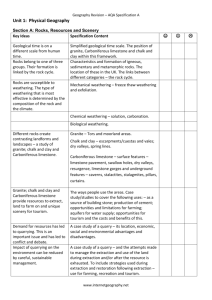
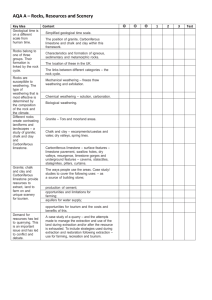
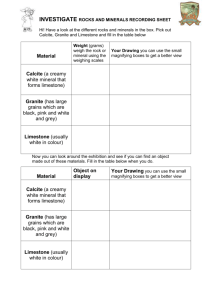
Chapter 5 Soluble rocks

Chapter 5 Soluble rocks
Solubility – threatens water storage and water conveyance projects with sever problems involving potential leakage and ground collapse
Question:
What is the most common soluble rock type?
Question:
What are the 3 groups or classes of limestone?
The divisions are based on their mode of formation?
– Biochemical
– Chemical
– Detrital
Biochemical limestone
Rocks formed from living organisms – shells of microscopic planktonic foraminifera and plates of calcareous algae
Calcareous algae
Upper Tertiary
Dhofar, Southern
Oman
Ammonite and tubiphythes in "Treuchtlingen Marble"; trade name: Jura Gelb
Upper Jurassic (Malm Delta)
Treuchtlingen, Germany
Oolith
Lower Aptian
Near Doman, Recita Zone, Southern Carpaten, Rumania
Belemnite and tubiphythes "Treuchtlingen Marble"
Upper Jurassic (Malm Delta)
Treuchtlingen, Germany
Sponge
Devonian ?
Bucchan Caves?, Australia
Belemnite battlefield
Lower? Jurassic
Mistelgau, Northern Bavaria, Germany
fossiliferous limestone, very rich in crinoids; trade name:
Derbyshire Fossil
Carboniferous
Coahill, Derbyshire, England, Great Britain
Beige limestone with rounded intraclasts and fossils (gastropods, corals)
Red limestone with bivalve shells and other molluscs, brachiopods?
Helvetikum
Grünten, Allgäu, Germany
Recrystallized stromatopore-reef limestone (Lahn-Marble) with stromatopores, crinoids und tabulate corals (Thamnopora or Heliolites); grey ruditic lime is normal background sedimentation; red is storm sediment
Middle Devonian
Bongard-Quarry (?), Villmar, Kreis Limburg-Weilburg, Germany
Reddish coral limestone
Carboniferous
Avon Gorge, Bristol, UK
Brown-reddish coral limestone
?, probably Carboniferous or Devonian
Beach fortification near the bunkers from WWII, Dunkerque,
France
Biochemical limestone
• Bedded and jointed
• Hardness 3 to 4, mineral calcite and dolomite
Chert horizons
• hardness 6 - silica
Chert concretion from Boom
Clay Belgium
Chert
cherts are formed from the tiny (0.5 to 1.5 mm) silica shells of radiolaria.
Chalk
• one unusual example of biochemical limestone is chalk – compacted but not lithified
Chalk
• white friable and very porous
• shells of microscopic planktonic foraminifera and calcareous algae
Chalk
• massive uniform layers or
• very thick beds separated by shale partings
• not typical to be jointed as is most limestone
Horizons of chert concretions common
Chalk
Chalk
Other names dependent upon content of clay and chalk
Chalk > 95% CaCO3
Clay Chalk >5%<13% clay
Clay Marl >13%<25% clay
Calcareous mudstone >25%clay
Chemical limestone
Precipitate of calcite CaCO3
(uncommon), occurs in warm CaCO3 rich seas oolites.
Oolites
• Concentric radial structure
1. Precipitation of CaCo3
sea water is almost saturated in CaCO2 – decrease in the content of CO2 by warming or by the action of plants in shallow water can cause calcium carbonate to precipitate
2. Precipitation of CaCo3
rivers saturated in CaCO2 precipitate it when they enter saline environments, called travertine
3. Precipitation of CaCo3
groundwater saturated in CaCO3 precipitates it when the groundwater emerges into the atmosphere, springs called tufa brick in a church
4. Precipitation of CaCo3
evaporation in arid and semiarid regions leads to the precipitation of CaCO3 called caliche
Detrital limestone
• particles of CaCO3 cemented together, very porous
• Names are dependent upon the size and nature of the particles
– clay – calcilutite
– sand – calcarenite
– gravel – calcirudite
– shell fragments – coquina or shell-hash limestone
calclutite
calcarenite
• sand size grains of CaCO3
calcirudite
Question:
• Compare the strength of calcarenite with orthoquartzite with respect to their particles, and cement.
• What is the expected difference in porosity?
Dolostone or Dolomite
recrystallized limestone which contains
Mg
• composed 90% of the mineral dolomite,
• less soluble than calcite
• composition changes after deposition
– type of chemical re crystallization
Dolostone or Dolomite
dolomitization is not always uniform
Dolostone or Dolomite
Fractures in the dolostone bedrock conduct groundwater
Dolostone or Dolomite
Mountain range called dolomites
Marble
What type of rock is it?
Marble – metamorphic rock formed from limestone – complete recrystallization
Evaporate rocks
• Gypsum CaSO4 2H2O
• Anhydrite CaSO4
• Halite NaCl
Evaporate rocks
• Gypsum CaSO4 2H2O
• Anhydrite CaSO4
• Halite NaCl
Evaporate rocks
• Gypsum CaSO4 2H2O
• Anhydrite CaSO4
• Halite NaCl
Gypsum
• massive or bedded
• associated with rock salt, shale, dolomite and limestone
• bituminous material common
• often intensely folded and brecciated – due to its formation: Anhydrite + hydration results in Gypsum and EXPANSION and deformation
• highly soluble – 170 times more soluble than calcite but only 1% that of NaCl
• lacks strength for caverns to form
Anhydrite
• stable form of CaSO4 above 43 degrees
• stable at any temperature when there is no H2O present
• Hydration – volume expansion of 35%
• Hydration depth is less than 150 m (fig
5.9)
• Hydration changes the anhydrite to
Gypsum
• 3.5 Mpa pressure due to hydration
Halite or Rock Salt
• massive beds with inclusions of brine
• salt dome formation – diapirs Fig. 5.8 – intrusions of salt into overlying rocks
• salt domes – up to 3 km diameter
• steep and vertical joints
• impermeable – trap for oil
• cap rock deformed
• source as much as 5 km deep
• salt diapirs that pierce the ground become salt glaciers
salt dome
Salt glacier
Solution processes and effects
Common in limestone, dolostone and marble
Stages of Karstification
Youth
Maturity
Old age
Stages of Karstification
Youth
Maturity
Old age
Two kinds of subsidence
• dissolved
– slow subsidence sinkholes
– densification of sediments
• collapsed – p166 fig 5.17, 5.16
– loss of support triggered by:
• lowered groundwater level
• heavy rain storms – wash out of sediments
• vibrations
• increased infiltration
Two kinds of subsidence
• dissolved
– slow subsidence sinkholes
– densification of sediments
• collapsed – p166 fig 5.17, 5.16
– loss of support triggered by:
• lowered groundwater level
• heavy rain storms – wash out of sediments
• vibrations
• increased infiltration
Geologic Controls on the
Formation of Karst
Cavities occur in almost all soluble rocks – but their size and shape is dependent upon the composition, texture, and structure of the rock, its strength and its geological history
Residual Soils
limstone gives terra rosa a soil
• red due to the high content of hematite and limonite, FeO;
• clay rich and fissured thus well drained
Residual Soils
dolominte gives a soil called wad
• rich in magnesium rich minerals such as clorite and montmorillonite
• are highly compressible and swelling
• Natural water content of more than
200% (greater than bentonite)
Volcanic tuff montmorillonite
Engineering properties
Case study
Vajont slide in Italy
Engineering properties
Exploration targets and problems
• subsurface cavities and sinkhole areas
– location
• determination of the surface of the solid rock below the residual soil, top of rock
(SAME for Sweden)
• location of highly soluble layers
• gypsum – drilling to determine occurrence
• anhydrate – gypsum contact
Water supply
• water plentiful but the system is very sensitive to pollution
• water will result in CaCO3 deposits on pipes
Rock salt
impermeable – proposed site for deposit of burnt atomic fuel
Foundation
• karsts – each bearing point must be studied
• cavities – collapse potential must be studied – drill plan dependent upon the risk
• pinnacle rock top
§ differential settling
§ differential support
§ pinnacles undermined
§ piles - glide off pinnacle
Foundation
• gypsum and water leads to settling, collapse and solution
• anydydrate leads to heaving
• calcarenite and chalk have limited bearing capacity
• weathered products extremely compressable
Dams and Reservoirs
• All the same problems as mentioned for Foundations above
Dams and Reservoirs
• IMPOUNDMENT of WATER not obvious
• no lake may form if water flows through the ground
• dissolve new channels
• washout of old channels
• dissolution of gypsum and salt
• foundation stability endangered by clay seams
• high pore pressures can occur if the foundation is located on an upwardly discharging spring
Tunneling
• limestone
– relatively strong – caverns with considerable size can form naturally in them
– karsts – are a problem – collapse and sudden inflow of water
Tunneling
• evaporate rocks
– salt
· massive - good, bedded – poor
· easily dissolved
· organic materials common – risk for explosions
· oils and gas outbursts
– gypsum
· fractured
· disturbed bedding and voids
· squeeze common
· dissolvable
Tunneling
• chalk, calcarentites, and cacirudites
– weak – requires additional supports due to collapse risk
– >15 Mpa >300 m plastic ductile deformation
– <300 m elastic brittle deformation
Materials of construction
Aggregates:
• limestone and dolomite – good in both asphalt and concrete given a reasonable strength
• they give good particle shape
• good particle size distribution
• NOTE – strength in asphalt is not sufficient for cold climates where studded tires are used
• >15% argillite not good
Materials of construction
Aggregates:
• chert – reactive in concrete and fractures in extreme cold
• gypsum is a SO4 – not allowed in concrete!! too weak for asphalt
Materials of construction
Dimention stone
• limestone, dolomite and marble are all very common – not always good on exteriors (warping)
Case Study
Failures and Near Misses from surface
Collapse over Cavities
Sinkholes associated with lowered ground water table
West Driefontein mine – south African mine
• increased rate of ground-water with drawl caused the main surface stream to go dry
• cavern had developed
• 117m deep boring in residual soils
• grouted in 171 holes, 9-15 m deep
• surface paved to prevent infiltration around the plant 60 m in all directions
• the entire crushing plant disappeared into a sinkhole – with 29 people – never found
• the hole was 55 m in diameter and more than 30 m deep
South Africa train 1975 – near the Driefontein mine
• ground water lowered in the Dolomite
• railroad was closed for passenger trains over a year during which remedial measurements were taken
• 8 days after the rout was re opened a sinkhole formed
• the train driver could not stop the train in time
• 3 coached derailed – 2 left hanging over the sinkhole
Failure of the Tarpon
Strings Bridge – Florida
• 1969 – 3 foundation units were swallowed into a sinkhole
• the railroad traffic did not stop in time – one person was killed
Palermo airport, Sicily
• cavity of 12,000 m3 volume
• 2 m below the pavement
• cavity extended over the entire width of the runway just at the place where aircraft touch down
• cavity was plugged with concrete through holes drilled from the pavement
Kentucky Dam
• 10 potential sites studied
• bedrock was flat lying limestone – overlain by 30m of tertiary sediments – overlain by cherty residual soil
• karsts in the limestone
• weathered down 95 m (Fig. 5.23)
• solution more intense at changes in bedding orientation due to the higher frequency of joints
Kentucky Dam
• both dam abutments were situated on thick sequences of soils and alluvium
• bedrock had numerous caves 65 m deep and 18 m wide
• most partially filled with residual clay
• solution cavities were very continuous laterally along certain beds with unstable minerals
Kentucky Dam
solved
• drillings were made so miners could go down and clean the cavities of clay and soil
• the cavities were filled with grout material
• this formed an underground cutoff
• 50 km of diamond core holes
• 2.3 km of calyx holes
• 20,000 m3 grout
Great falls dam
• horseshoe formed river
• leakage through the divide of 10 % the capacity
• leakage increase by 1% per year
• the lake level was lowered and 96 inlets were detected
• trace elements were used to trace the flow
• these were then grouted and leakage cut off
UCSC
• Karstic limestone where an Olympic size swimming pool was to be constructed
• Relocated to miss karts filled with silt –
• Since this is an earthquake area, near the
San Andreas Fault Zone, liquefaction would be probable over silt
• new location in a collapsed dolline
• the base was filled, lined and under the liner a collector for leakage water installed
Grout Curtain at El Cajon
Dam
• thin arch dam 238 m high to be built in karstic area overlain with volcanics and 4 major faults in the area
• drillings revealed caves 200 m lateral extent
• grout curtain made in form of a bathtub
• 514 km of drillings
• 14 km galleries
• grout curtain area 530 000 m2
• 2 ½ years to complete
• 10,000 m3 cave detected during exploration resulted in the relocation of the bathtub grout curtain
New Mexico Mc Millan reservoir 1893
• gypsum beneath the reservoir abutment
• soon after filling the cliff began to crack and collapse
• 1909 embankment built parallel with the cliff to cut off the contact with the water
• 12 m subsidence
• 1942 underground caves and chanels 60 million m3
Oklahoma 1965 – 11 m high dam
• soon after construction a sinkhole formed
• volume of 2 000 m3 under the spillway
Pollution in karst – Australia
– Mount Gambier,
• town with 20 000 inhabitants located on limestone
• The karstic limestone aquifer overlies a clay bed which is impermeable (aquiclude) which in turn overlays a delta mollase aquiclude
• The upper aquifer is polluted with waste from industry and sewage
• The lower aquifer is the source of ground water
Pollution in karst – Australia
– Mount Gambier,
• the state of leakage between the two aquifers is threatened
• if the amount of ground water pumped out of the lower aquifer exceeds the infiltration there is a risk that the direction of leakage through the aquiclude will change so leakage will be from the upper polluted aquifer down to the confined aquifer
• today the lower aquifer is artesian and under high pressure but if the pressure gradient is lowered this will change