Assignment no. 5
advertisement
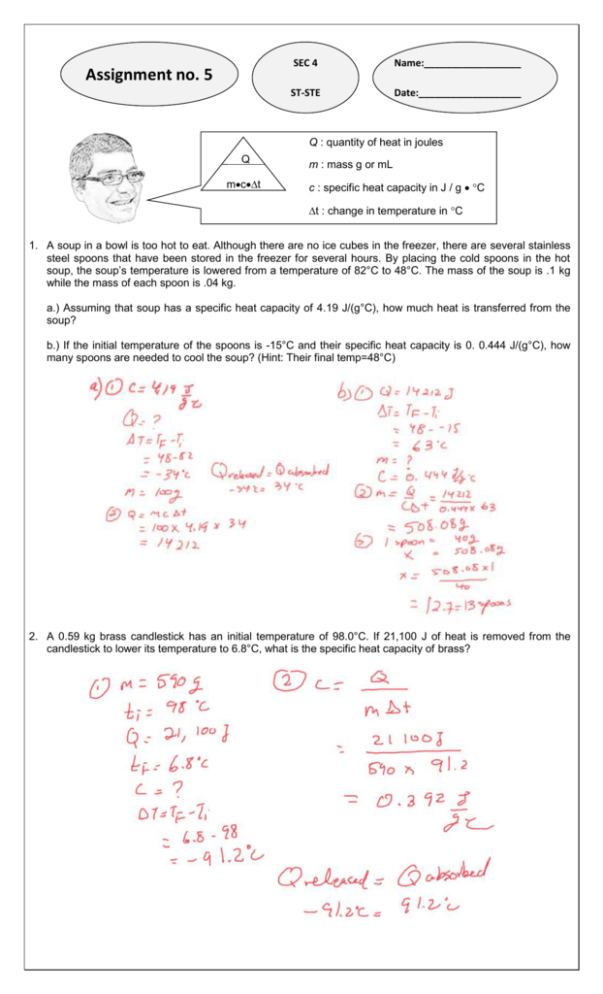
Assignment no. 5 SEC 4 Name:__________________ ST-STE Date:___________________ Q : quantity of heat in joules _ Q mct . m : mass g or mL c : specific heat capacity in J / g C t : change in temperature in C 1. A soup in a bowl is too hot to eat. Although there are no ice cubes in the freezer, there are several stainless steel spoons that have been stored in the freezer for several hours. By placing the cold spoons in the hot soup, the soup’s temperature is lowered from a temperature of 82°C to 48°C. The mass of the soup is .1 kg while the mass of each spoon is .04 kg. a.) Assuming that soup has a specific heat capacity of 4.19 J/(g°C), how much heat is transferred from the soup? b.) If the initial temperature of the spoons is -15°C and their specific heat capacity is 0. 0.444 J/(g°C), how many spoons are needed to cool the soup? (Hint: Their final temp=48°C) 2. A 0.59 kg brass candlestick has an initial temperature of 98.0°C. If 21,100 J of heat is removed from the candlestick to lower its temperature to 6.8°C, what is the specific heat capacity of brass? 3. Mercury has one of the lowest specific heat capacities. If 257J of heat are added to .45 kg of mercury, the mercury’s temperature will increase by 4.09ºC. What is the specific heat capacity of mercury? 4. A 0.38 kg drinking glass is filled with a hot liquid. The liquid transfers 7032 J of heat to the glass. If the temperature of the glass increases by 22ºC, what is the specific heat capacity of the glass? 5. The temperature of air in a foundry (Metal casting factory) increases when molten metals cool and solidify. Suppose 9 900 000J of heat is added to the surrounding air by the solidifying metal. The air’s temperature increases by 55 ºC, and the air has a specific heat capacity of 1.02 J/(gºC) What is the mass of the heated air? 6. A 0.225 kg sample of tin, which has a specific heat capacity of 0.21 J/(gºC) is cooled in water. The amount of heat transferred to the water is 3900 J. If the final temperature of the tin is 18°C, what was the initial temperature? (Hint: Find the change in temperature first.) 100.54 degrees Celsius 7. Randy has a 500 g of water at 20°C. If he wants the final temperature of the water to be 75°C, how many Joules of heat will he need to add? 8. If 216 J of energy is required to raise the temperature of aluminum from 15°C to 35°C, calculate the mass of aluminum. (Specific heat of aluminum is 0.90 J/( g°C) 9. 50 Joules of heat are added to a 2 kg block of iron. If the specific heat of iron is 0.444 J/(g°C), how much will its temperature increase? 10. .The initial temperature of 150 g of ethanol was 22°C. What would be the final temperature of the ethanol if 3,240 J of energy were added? The specific heat capacity of ethanol is 2.44 J/ (g°C) (Hint: Find the change in temperature first.)









