Stoichiometry Lab
advertisement

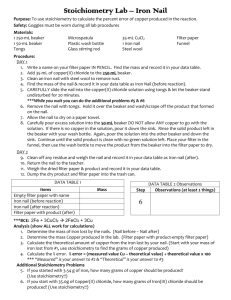
Stoichiometry Lab Prupose: to determine the values for coefficients used in a balanced chemical equation. Materials: Goggles!! 50-mL graduated cylinder 1 250-mL beaker 1 100-mL beaker Tongs Spatual Plastic wash bottle Glass stirring rod 35-mL CuCl2 1 iron nail Steel wool Procedure: DAY 1 1. Determine the mass of your 100-mL beaker, record it in your data table, and set this beaker aside. 2. Add 35-mL of copper(II) chloride to the 250-mL beaker 3. Clean an iron nail with steel wool to remove rust. 4. Find the mass of the nail & record it in your data table. 5. CAREFULLY slide the nail into the copper (II) chloride solution & let the beaker stand undisturbed for 20 minutes. 6. Use crucible tongs to remove the nail. Hold the nail over the beaker and wash/scrape off the product that formed on the nail. 7. Allow the nail to dry on a paper towel. 8. Pour the green solution with the solid product into the 100-mL beaker. Then, carefully decant the liquid portion back into the 250-mL beaker leaving only the solid product in the 100-mL beaker. 9. Dispose of the green solution in the sink. 10. Use the wash bottle to wash the product in the 100-mL beaker and again decant the wash water into the other beaker. Repeat a couple of times. 11. Give the 100-mL beaker containing the solid product to the teacher to be dried. DAY 2 12. Determine the mass of the nail (after) & record it in your data table. 13. Return the nail to the teacher. 14. Determine the mass of the 100-mL beaker with the dry product & record it in your data table. 15. Dump the dry product into the trash can and rinse out the beaker. DATA TABLE 1 Mass Determinations DATA TABLE 2 Items Mass Visual Evidence of a Chemical Reaction Empty dry 100-mL beaker Step Observations (at least 2 things) Between Iron nail (before reaction) 5-6 Iron nail (after reaction) 100-mL beaker & dry product Analysis & Conclusion (show ALL work for calculations—otherwise complete sentences!) 1. Write a balanced chemical equation for this reaction. 2. Classify the type of reaction. 3. Determine the mass of iron lost by the nails. 4. Determine the mass Copper produced in the lab. 5. Calculate the theoretical yield of copper given the iron lost by your nail 6. Calculate the % error. % error = (measured value – accepted value) ÷ accepted value x 100 Additional Stoichiometry Problems 7. If you started with 3.54 g of iron, how many grams of copper should be produced? (use stoichiometry!!) 8. If you start with 35.0g of Copper(II) chloride, how many grams of Iron(II) chloride should be produced? (use stoichiometry!!)