Chapter_7_The_Complement_System (1)
advertisement
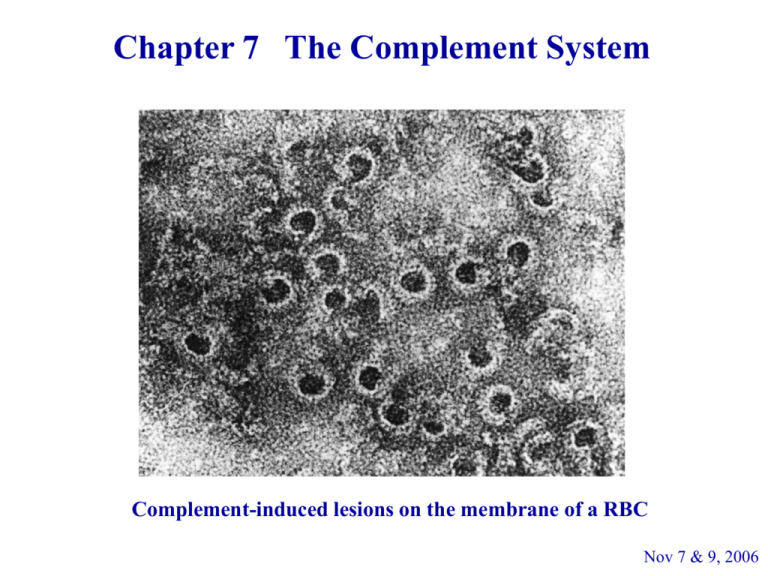
Chapter 7 The Complement System Complement-induced lesions on the membrane of a RBC Nov 7 & 9, 2006 Discovery of Complement 1890s Jules Bordet (Institut Pasteur in Paris) observed: 1. Sheep antiserum to the bacterium Vibrio cholerae caused lysis of the bacteria. 2. Heating the antiserum destroyed its bacteriolytic activity. 3. Addition of fresh normal serum, that contained no Abs against the bacterium and was unable to kill the bacterium by itself, restored the ability to lyse the bacteria by the heated antiserum. Q1: If you were Bordet, what would you hypothesize? A1: Bacteriolytic activity requires 2 different substances. a. the specific antibacterial Abs, which are resistant to the heating process b. heat-sensitive component responsible for the lytic activity c. 56˚C, 30 min will inactivate complement Paul Ehrlich in Berlin carried out similar experiments and named the substance complement defining it as “the activity of serum that completes the action of Ab.” Bordet won the Nobel Prize in 1919 – Complement-mediated bacteriolysis Complement Plays a Key Role in Both Innate and Adaptive Immunity - The complement system is the major effector of the humoral branch of the immune system. - Although the discovery of complement and most early studies were linked to the activity of complement following Ab binding, a major role for this system is the recognition and destruction of pathogens based on recognition of pathogen-associated molecular patterns, or PAMPs, rather than on Ab specificity. The Complement Components - More than 30 soluble and cell-bound proteins - Participate in both innate and adaptive immunity - Produced by hepatocytes (mainly), monocytes and epithelial cells of the gastrointestinal and genitourinary tracts - Constitute 5% (by weight) of the serum globulin fraction - Many components are proenzymes (zymogens), which are functionally inactive until proteolytic cleavage, which removes an inhibitory fragment and exposes the active site. - Reaction starts with an enzyme cascade. The Functions of Complement 1. Lysis of cells, bacteria, and viruses – the major effector of the humoral branch of the immune system 2. Opsonization, which promotes phagocytosis of particulate Ags 3. Binding to specific complement receptors on cells of the immune system, triggering specific cell functions, inflammation, and secretion of immunoregulatory molecules 4. Immune clearance, which removes immune complexes from the circulation and deposits them in the spleen and liver Complement Activation 1. Classical Pathway – begins with the formation of Ag-Ab complex 2. Alternative Pathway – is initiated by cell-surface constituents that are foreign to the host – Ab-independent 3. Lectin Pathway – is activated by the binding of mannosebinding lectin (MBL) to mannose residues on glycoproteins or carbohydrates on the surface of microorganisms – Ab-independent Classical Pathway Begins with Ag-Ab Binding C1 molecule soluble Ag-Ab* or bacteria-Ab* ↓ conformational changes in the Fc portion of Ig ↓ expose a binding site on the CH2 domain of the Fc portion for the C1 component of the complement system * complement-activating Abs: IgM, IgG1, IgG2, IgG3 (human) C1qr2s2 stabilized by Ca++ C1q binds to Ag-bound Ab ↓ Binding of C1q to Fc induces a conformational change in C1r ↓ __ C1r converts to an active serine protease enzyme, C1r, __ which cleaves C1s to a similar active enzyme, C1s CH2 domain of the Ab bacterium __ C1s has two substrates, C4 and C2 __ ↓ C1s hydrolyzes C4 into C4a and C4b, and hydrolyze C2 into C2b and C2a ↓ ___ C4b and C2a form a C4b2a complex, also called C3 convertase, referring to its role in converting the C3 into an active form. an anaphylatoxin*, or a mediator of inflammation * Anaphylaxis C2a C4b ( ) ____ C4b2a (C3 convertase) hydrolyzes C3 into C3b and C3a ☺ ↓ ____ ______ C3b binds to C4b2a and form C4b2a3b (C5 convertase) _______ ↓ C4b2a3b cleaves C5 into C5b and C5a opsonization inflammatory responses inflammatory responses © _____ Hydrolysis of C3 by C3 Convertase C4b2a formation of a labile internal thioester bound in C3 bind to free – OH or – NH2 groups on a cell membrane generates > 200 C3b ☺® Bound C3b exhibits various biological activities, e.g., binding of C5 and binding to C3b receptors on phagocytes. The Alternative Pathway Is Ab-independent - The activation of alternative pathway doesn’t need Ab; thus, it is a component of the innate immune system. - It is initiated by cell-surface constituents that are foreign to the host, e.g., bacterial cell wall. - C1, C4 and C2 are not involved in the alternative pathway. - Four serum proteins, C3, factor B, factor D, and properdin, are involved in this pathway. plasma C3, with an unstable thioester bond, can be hydrolyzed spontaneously into C3a and C3b. C3b attaches to the surface of bacteria, yeasts, viruses (or even host’s own cells ®). Mg++ Ba (stabilization of C3bBb) ___ analogous to the C4b2a complex in the classical pathway © The Lectin Pathway Originates with Host Proteins Binding Microbial Surfaces Lectin: proteins that bind to a carbohydrate MBL (mannose-binding lectin): - an acute phase protein which binds to mannose residues on glycoproteins or carbohydrates on the surface of microorganisms (structurally similar to C1q) MASP-1 & MASP-2: MBL-associated serine protease (structurally similar to C1r and C1s) - MBL is induced during inflammatory responses. - After MBL binds to the surface of a microbe, MBL-associated serine proteases, MASP-1 and MASP-2, bind to MBL. - The MBL-MASP-1/2 complex mimics the activity of C1r and C1s, and causes cleavage and activation of C4 and C2. - Thus, the lectin pathway is Ab-independent. It is an important innate defense mechanism comparable to the alternative pathway, but utilizing the elements of the classical pathway, except for the C1 proteins. Q2: Why doesn’t mannose-binding lectin (MBL) bind to host carbohydrates? A2: Mammalian cells normally have sialic acid residues covering the sugar groups recognized by MBL and are not a target for binding. The Three Complement Pathways Converge at the Membrane-attack Complex The three complement pathways converge at the production of an active C5 convertase ↓ C5b6789 membrane-attack complex (MAC) Formation of C5b6789, Membrane-attack Complex C5b attaches to C6, then to C7, and the C5b67 complex inserts into the membrane. ↓ binding of C8 to membrane-bound C5b67 induces a 10 Å pore. ↓ binding and polymerization of C9, a perforin-like molecule, to C5b678 ↓ The completed membrane-attack complex (MAC) has a tubular form and functional pore size of 70 – 100 Å Lysis of an E. coli by Complement Strict Regulation of the Complement System - Discrimination between microorganisms and self - Passive mechanisms of regulation : highly labile components that undergo spontaneous inactivation if they are not stabilized by reaction with other components - A series of specific regulatory proteins: inactivate various components a b c d e f g h i j (B) ____ C4b2a ____ C3bBb C3 convertase b c f (C) h i j Biological Effects Mediated by Complement 1. Cell lysis The membrane-attack complex can lyse a broad spectrum of cells: G(-) bacteria parasites viruses erythrocyte nucleated cells (tumor cells) Because the activation of alternative and lectin pathways is Ab-independent, these pathways serve as important innate immune defenses against infectious microorganisms. 2. Inflammatory response - Various peptides generated during activation of complement play a decisive role in the development of an effective inflammatory response. - C3a, C4a, C5a (called anaphylatoxin) bind to complement receptors on mast cells and basophils and induce degranulation with release of histamine and other mediators. - The anaphylatoxins also induce smooth-muscle contraction, increased vascular permeability, extravasation, and chemoattraction (induced by C5a, C3a, and C5b67) 3. Opsonization - C3b is the major opsonin of the complement system, although C4b and iC3b also have opsonizing activity. binds to the surface of microbes C3 convertase Opsonization by Ab and complement 4. Viral neutralization - Formation of larger viral aggregates reduces the net number of infectious viral particles - The deposits of Ab and complement on viral particle neutralizes viral infectivity by blocking attachment to susceptible host cells and facilitates binding of the viral particle to cells possessing FcR or CR1. 5. Clearance of immune complexes