W14 Photosynthesis W14 Photosynthesis
advertisement
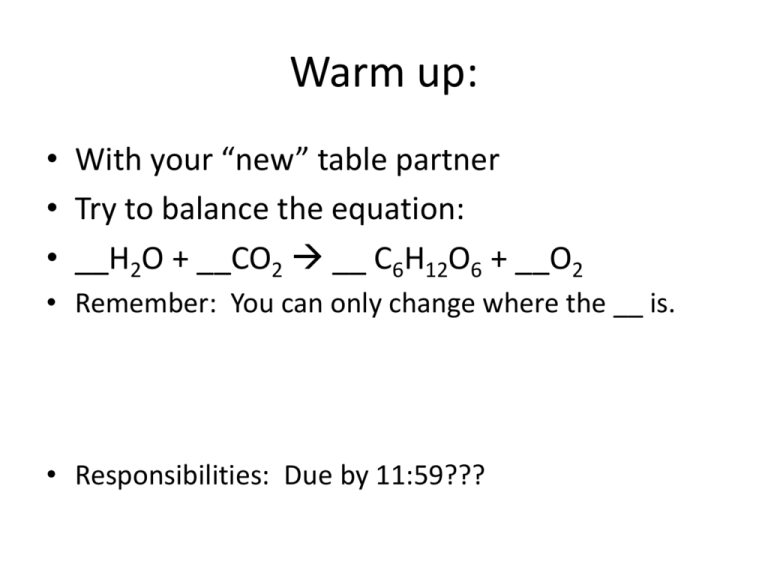
Warm up: • With your “new” table partner • Try to balance the equation: • __H2O + __CO2 __ C6H12O6 + __O2 • Remember: You can only change where the __ is. • Responsibilities: Due by 11:59??? ATP • The basic unit of useable energy that cells need to power their lives is known as ATP. ATP stands for: Adenosine Tri-phosphate. • ATP is “the money” of the cell. Mitochondria is responsible for using this. • It is the bonds that hold the Adenosine, Ribose (sugar) and Phosphate groups together that hold the energy. When the molecule is broken apart by cells, this energy is released and used. • ATP – Adenosine Tri-phosphate • ATP = raw energy. Photosynthesis • Photosynthesis is an ‘energy storing’ process. Performed by plants (and some bacteria), it harnesses the energy available in sunlight for the purpose of building glucose, a basic sugar molecule organisms use as food. The term comes from the two latin roots ‘photo’ always meaning light, and ‘synthesis’ which means to build. • The essential factor plants have that animals do not, allowing them to perform this function, is the pigment chlorophyll. PSN • Uses Sunlight to make “glucose” • The essential chemical reaction for life. • Happens in chlorophyll PSN Where is it happening? • Chlorophyll acts like an energy trap, or a rechargeable battery. It collects the energy available from sunlight and then uses that to begin the chemical process that forms glucose. • Collects Energy 2 Phases of Photosynthesis • Light Reactions:. • Water is split into oxygen and hydrogen. • The oxygen is given off as a waste product, the hydrogen is used by the plant. • Keep the H • Release the O Stronger, clearer • Use you notes and diagram to write a summary of the light dependent reaction. • Go meet up with another person. Each person will share their summary while the other takes notes. Steps of light reaction 1. Happens in thylakoid inside a cholorplast. 2. Accept a photon (light). 3. Split water – Use H to make NADPH and ATP – Lose O as O2 Steps of the Dark Reactions 1. Carbon dioxide is fixed by RDP (chloroplasts contain ribulose diphosphate or RDP. This is a 5-carbon sugar molecule with two phosphate groups. This is a carbon dioxide receptor. 2. PGA combines with hydrogen and forms PGAL. 3. Glucose is formed. Steps of dark reaction 1. Inside the stroma (chloroplast goo) 2. Use energy to make sugar in the calvin (dark) cycle. 3. ATP and NADPH are changed to ADP and NADP+ and go back to the light reaction where they are used again. • https://www.youtube.com/watch?v=mYbMP wmwx88 Want a headache from a nice person? • https://www.youtube.com/watch?v=FfLLHQD gpjI MAJOR IDEA!!!! • Photosynthesis is the bridge between the organic (living) and inorganic (nonliving)world! This is the process in which nonliving building blocks are organized into a carbon based substance that then powers life. The Screaming Gummy My only regret is that I have but one life to give to CODALAND! Materials: • • • • • one 25x150 mm Pyrex test tube one ring stand with clamp for test tube 5 grams potassium chlorate Bunsen burner one candy gummy bear CAUTIONS! • Caution! This reaction produces a large quantity of heat, flame, and smoke (mostly water vapour). It should be done in a well ventilated room, or, preferably, in a fume hood. Potassium chlorate should be used with caution. It is a strong oxidizing agent, especially when molten. Keep all combustible materials away from the reaction area. Make sure the test tube used is scrupulously clean and the mouth is pointed away from the audience. GOGGLES! Procedure: 1. Set up the stand and clamp, and support the test tube in the clamp in a vertical position. 2. Add 5-7 grams of potassium chlorate to the test tube (about 1 cm in depth). 3. .Gently heat the tube with the burner until the potassium chlorate is completely molten. Bubbles of oxygen will begin to form. 4. .Remove the burner and use crucible tongs to drop in the gummy bear, and stand back! Analysis • • • • • • • • • • • • • • • • Student Analysis: What is a decomposition reaction? Write a balanced reaction for the decomposition of potassium chlorate. How could we test for the presence of oxygen in this decomposition reaction? Why is it necessary to heat this reaction? What is the difference between an endothermic and an exothermic reaction? Write a balanced reaction for the oxidation of glucose. Show, using oxidation numbers, which substance is oxidized and which substance is reduced in the reaction. Also state the oxidizing agent and the reducing agent. What caused the Gummy Bear to explode? Observe the products of this reaction. Did the reaction go to completion, forming only CO2 and H2O? What would the products include if the combustion was incomplete?