Acid Rain and Photochemical Smog
advertisement

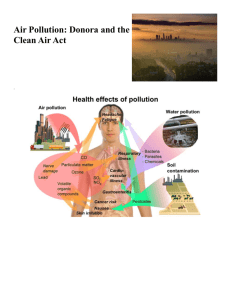
Choi Man Ho Chan Kwun Keung Acid Rain and Photochemical Smog What is Acid Rain? • Unpolluted rainwater is slightly acidic (pH 5.6) because of the carbon dioxide from air dissolved in it. CO2 + H2O => H2CO3 • Rainwater with a pH as low as 2.5 has been recorded in some parts of the world. They are commonly known as acid rain. What is Acid Rain? • "Acid rain" is also a broad term used to describe several ways that acids fall out of the atmosphere. • A more precise term is acid deposition, which has two parts: wet and dry. • Wet deposition: acidic rain, fog, and snow. • Dry deposition: acidic gases and particles Causes of Acid Rain • Sulphur dioxide (SO2) and nitrogen oxides (NOx) are the primary causes of acid rain. • Acid rain occurs when these gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds. • Sunlight increases the rate of most of these reactions. The result is a mild solution of sulfuric acid and nitric acid. Causes of Acid Rain Causes of Acid Rain Formation of Sulphuric Acid (H2SO4) • Sulphur dioxide (from the burning of fossil fuels in power plants and industrial plants) is the primary cause of acid rain. • In the atmosphere, sulphur dioxide is slowly oxidized to sulphur trioxide which dissolves readily in water droplets to form sulphuric (vi) acid. • 2SO2+O2 => 2SO3 • SO3 + H2O => H2SO4 Formation of Sulphuric Acid (H2SO4) • The actual pathways are more complex. • The formation of SO3 from SO2 is influenced by the prevailing atmospheric conditions: sunlight, temperature, humidity, and the presence of hydrocarbons, nitrogen oxides and particulates in the atmosphere. Formation of Sulphuric Acid (H2SO4) • Sulphuric (iv) acid is also formed when SO2 dissolves in rainwater: • SO2 + H2O => H2SO3 Formation of Nitric Acid (HNO3) • Nitrogen oxides (from the burning of fossil fuels in automobiles and power plants) also cause the formation of acid rain. • When released to the atmosphere, nitrogen monoxide combines with atmospheric oxygen to form nitrogen dioxide: • 2NO + O2 --> 2NO2 Formation of Nitric Acid (HNO3) • In a series of complex reaction, nitrogen dioxide combines with oxygen and water vapour to form nitric (v) acid. • 4NO2 + 2H2O + O2 --> 4HNO3 Environmental Problems • (i)In water of pH less than 4.5, calcium metabolism in fresh water fish will be affected, leading to poor health and stunted growth. • As a result, diversity and population of some fresh water species will be reduced. Environmental Problems • (ii)In soil of pH less than 4.5, absorption of cations by plants will be affected, resulting in death of plants. • (iii)Inflow of acidic water containing poisonous metal ions from soil will kill the fish and water plants • (iv)Acid rain corrodes metals and accelerates the deterioration of building, rock and statue. How do we Measure Acid Rain? • Acid rain's pH (and the chemicals that cause acid rain) is monitored by two networks, both supported by EPA. • The National Atmospheric Deposition Program measures wet deposition, and its Web site features maps of rainfall pH and other important precipitation chemistry measurements. How do we Measure Acid Rain? • The Clean Air Status and Trends Network (CASTNET) measures dry deposition. Its Web site features information about the data it collects, the measuring sites, and the kinds of equipment it uses. How do we Reduce Acid Rain? • Understand acid deposition's causes and effects • Clean up smokestacks and exhaust pipes • Use alternative energy sources • Restore a damaged environment • Be green How do we Reduce Acid Rain? • EPA's Acid Rain Program limits, or "caps," sulphur dioxide (SO2) emissions from power plants at 8.95 million tons annually, allows those plants to trade SO2 allowances, and reduces nitrogen oxide emission rates. What is Photochemical Smog? • Photochemical smog is a mixture of pollutants which includes particulates, nitrogen oxides, ozones, aldehydes, peroxyacetyl nitrate (PAN), unreacted hydrocarbons, etc. • A brownish haze and painful eyes are often indicators of photochemical smog. Nitrogen dioxide is responsible for the brownish colour of the haze. Causes of Photochemical Smog • The reactions that lead to the formation of photochemical smog are irritated by sunlight and involve hydrocarbons and nitrogen oxides emitted from automobiles, the combination of sunlight, the catalysis by particulates and the abundant pollutants present in modern cities provide favourable conditions for smog formation. Causes of Photochemical Smog • Nitrogen dioxide from automobile exhaust first absorbs sunlight and breaks down into nitrogen monoxide and reactive oxygen atom: • NO2 => NO + O Causes of Photochemical Smog • The oxygen atom reacts with other components of automobile exhaust (e.g. unburnt hydrocarbons) and those of the atmosphere (e.g. oxygen) in a series of complex reactions to produce a variety of lachrymatory and toxic chemicals (e.g. peroxyacetyl nitrate). Causes of Photochemical Smog • O + O2 => O3 • O + hydrocarbons => aldehydes O3 + hydrocarbons => aldehydes • NO2 + O2 + hydrocarbons => lachrymatory substances, including peroxyacetyl nitrate (PAN): CHCOOONO2 Formation of Nitrogen Oxides (NOx) • The most natural way of forming nitrogen oxides [NOx, where x may be 1 or 2] is lightning. • Atmospheric nitrogen reacts with nearby oxygen to form nitrogen monoxide. • N2 + O2 => 2NO Formation of Nitrogen Oxides (NOx) • Nitrogen monoxide further reacts with oxygen to form nitrogen dioxide. • 2NO + O2 => NO2 • But this only generate limited amount of nitrogen oxides. • The pollution caused can be accounted for the engines of vehicles and furnaces. Due to their high temperature, these gases are evolved easily. The great number of cars on Hong Kong roads deepens the problem. Formation of Ozone (O3) • Ozone [O3(g)] is a pale blue gas (exists in the stratosphere of our atmosphere as the ozone layer). • It is formed from atmospheric oxygen by the absorption of ultraviolet radiation of the right energy (wavelength 250 nm). O2 => 2O O + O2 => O3 Formation of Ozone (O3) • Ozone itself undergoes photodissociation with 215-295 nm ultraviolet radiation. O3 => O2 + O O+O3=>2O2 • Thus, ozone is constantly created and destroyed. The above reaction is responsible for the vital screening effect of ozone. Formation of Ozone (O3) • Ozone can also be formed in the lower part of the atmosphere. • It is formed by reactions between nitrogen oxides and hydrocarbons under sunlight, or by electric sparks which occur in car engines or electrical appliances like photocopiers. • In nature, the gas can be generated during lightning. Formation of Hydrocarbons • Hydrocarbons have both the elements carbon and hydrogen. Motors of cars do not always burn the fuel completely. There is tiny amounts of unburnt hydrocarbons in car exhaust. • Petrols and organic solvents are left unattended and exposed to air. Since those solvents are volatile in nature, these hydrocarbons pose danger to our health. Formation of Particulates • Particulates make up smoke. • They may be ashes from burning of fuels. • If fossil fuels (like coal and oil) are burnt, the tiny particules formed are mainly soot (carbon) from incomplete combustion. • Incineration plants, factories and diesel vehicles are sources of emission. Environmental Problems • (i)It can cause headaches, eye, nose and throat irritations, impaired lung function, coughing and wheezing. • (ii)It can cause rubbers and fabrics to deteriorate. • (iii)It can cause damage plants, leading to the loss of crops. Another Smog Characteristics Industrial smog Photochemical smog Typical city London Los Angeles Climate Cool and humid Warm and dry Pollutants SOx, particulates NOx, O3, aldehydes, PAN, etc. Another Smog Characteristics Industrial smog Photochemical smog Major sources Major effects on humans Industrial and Motor vehicles household burning of coal oil Irritation of lungs Eye irritation and throat Times when worst episodes occur Winter months (especially in early morning) Summer months (maximum effect around noon) Cases of Smog (photochemical smog ) • China's Pearl River Delta region is starting to suffer photochemical smog due to heavy air pollution in the cities. • Emissions from factories, power plants and the rapid growth of vehicles in cities are providing the raw materials for photochemical smog. • The first case of photochemical smog happened in Los Angles as early as in 1943. Cases of Smog (photochemical smog ) Pearl River Delta region Hong Kong Cases of Smog (Industrial smog) • On 9 December 1952, foggy conditions developed over London. • Being very cold, most houses kept fires burning, with coal as the major fuel. • The smoke from these fires mixed with the fog and was unable to disperse, resulting in a smog which persisted for 4 days. Cases of Smog (Industrial smog) • The pH of air during the Great London Smog was as low as 1.6. During this period some 4000 more people died than would expected at this time of the year. Most of these additional deaths were due to respiratory disorders. Cases of Smog (Industrial smog) Before After smog New York City How do we Reduce Photochemical smog? • To address the problem of photochemical smog, we have to reduce the emissions of NOx and VOC. • Reduce the emissions from motor vehicles. Sources of Information • Chem notes by Ng sir • lhs.hkcampus.net/~lhs-chem/acid rain.files/frame.htm • www.epa.gov/airmarkets/acidrain • http://hk.geocities.com/xavier114fch/03/03b.htm • http://resources.emb.gov.hk/envired/text/hkissue/e_m1_1_3.htm • http://en.chinabroadcast.cn/143/2004-113/116@164576.htm The End Thanks for paying attention