LPN-C - Faculty Sites
advertisement

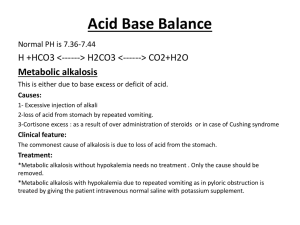
LPN-C Unit Three Fluids and Electrolytes Why are fluids and electrolytes important for the nurse to understand? Fluids and electrolytes are essential to identifying and defining the problem ◦ ◦ ◦ ◦ What is the relationship to the disease process What intervention is appropriate How will the intervention affect the patient Safety management of infusion therapy What is the IV infusion order Why was it ordered ◦ Continual assessment and evaluation of patient progress, status of labs, response to treatment Electrolyte imbalances can occur suddenly ◦ Must be able to assess changes ◦ Intervene appropriately and in a timely manner ◦ Frequent review of lab values, diagnostic tests, medications, IV fluid orders Homeostasis = a dynamic process involving a continuous series of selfregulating adjustments to maintain a balance of the internal environment ◦ Preserved through the intake and output of water ◦ Water is the primary chemical component within the body, and an individual can perceive a need for it Fluids Water Individuals with lean tissue mass have a higher percentage of body water than those with more fat The average adult female holds 52% of water by weight The average adult male holds 63% of water by weight Water serves as a vehicle for the delivery of electrolytes and nutrients to body cells Water serves as a vehicle for the excretion of waste products Water is a medium for biochemical reactions Water contributes to temperature regulation Water cushions organs and joints Water Intake The need for water is signaled through the mechanism of thirst ◦ Osmotic pressure from extracellular fluids ◦ Thirst center in the hypothalamus Percentages of daily water intake ◦ 60% water from drinking ◦ 30% water from moist foods ◦ 10% water from metabolism processes Water Output There are 4 avenues for daily water loss ◦ ◦ ◦ ◦ Lungs Skin Urine Feces The route of water loss depends on ◦ Temperature When the temperature outside is high, water loss via the skin and lungs increases ◦ Humidity ◦ Physical exercise Water Balance Urine output increases when water intake increases Urine volume decreases when water intake decreases, or when the body loses excessive water Water balance occurs when water intake equals water output Regulation of water balance ◦ Neurosecretions of the hypothalamus (antidiuretic hormone, or ADH/vasopressin) ◦ Mineralocorticoid secreted from the adrenal cortex (Aldosterone) Antidiuretic Hormone (ADH) ADH is produced by the hypothalamus Regulates water output by regulating extracellular fluid osmolarity Acts directly on the collecting ducts and tubules of the nephrons in the kidneys to bring about water reabsorption ADH ↓ Secreted by the posterior lobe of the pituitary gland ↓ Regulates water retention and excretion ADH (cont’d) Hypertonic extracellular fluid – Excess sodium or decreased blood volume ↓ Release of ADH ↓ Sensation of thirst and conservation of water in the body through reabsorption ADH (cont’d) Hypotonic extracellular fluid – Increased blood volume ↓ Pituitary signaled to inhibit the release of ADH ↓ Stimulates the excretion of urine ↓ Increases the concentration of extracellular fluid ADH (cont’d) Release of ADH can be influenced by drugs ◦ Increase of ADH Nicotine Morphine Barbiturates ◦ Inhibition of ADH Alcohol Malfunctions of the ADH system ◦ Diabetes insipidus ◦ Syndrome of inappropriate ADH (SIADH) Diabetes Insipidus -- Pituitary gland is unable to secrete ADH Not common (only 1 in 25,000 affected) ◦ Head trauma ◦ Surgery to the region of the pituitary and/or hypothalamus Urine is excessive and diluted ◦ Polyuria (urine output of 3-18 L/day) ◦ Polydipsia Hallmark signs include urine specific gravity at ≤ 1.005 and urine osmolality at <200/kg Treated with synthetic vasopressin PO or intranasal (desmopressin or DDAVP) Syndrome of Inappropriate ADH (SIADH) -Continued secretion of ADH Urine is concentrated and diminished Hyponatremia (<135 mEq/L) Increased urine sodium concentration (>20 mEq/L) Hypotonicity ◦ Plasma osmolality at <280/kg Water retention with increased extracellular fluid Closely monitor for weight change, fluid imbalance, restlessness, CHF, convulsions Treated with Lasix to maintain urine output and block secretion of ADH SIADH (cont’d) -Most common cause is idiopathic Other causes include ◦ Problems with the brain or head Trauma, hemorrhage Tumor, abscess Hydrocephalus, encephalitis Meningitis ◦ Medications Antibiotics, oral hypoglycemics, thiazide diuretics ◦ Stroke ◦ Respiratory issues Asthma, COPD, pneumonia Neonatal hypoxia Lung cancer, tuberculosis Aldosterone Regulates extracellular fluid volume ◦ Maintains water balance through sodium reabsorption in the nephrons ◦ Causes sodium retention (and subsequent water retention) if renal blood flow decreased Decrease in sodium level or extracellular fluid volume ↓ Secretion of Aldosterone ↓ Kidney reabsorption of water and sodium ↓ Increase in extracellular fluid Water Distribution The total volume of water in the body is distributed among two large compartments, which are separated by a selectively permeable cell membrane ◦ Intracellular compartment ◦ Extracellular compartment Nurses must understand the differences between these two compartments, and know how various illnesses and diseases can bring about imbalances Intracellular fluid is fluid that is contained within the cells of the body, and comprises 2/3 of the body’s total fluids Water Distribution (cont’d) Extracellular fluid is found outside the cells ◦ Comprises 1/3 of total body fluids ◦ High in oxygen and carbon dioxide ◦ Contains essential substances Glucose for energy supply Amino acids and fatty acids for growth, repair, and health maintenance Sodium, calcium, chloride, and bicarbonate ◦ Transports cholesterol, urea, lactate, creatinine, and sulfates ◦ Constant movement within the systemic circulation ◦ Main function is to maintain cell membrane permeability and to serve as a vehicle for movement of life-sustaining substances Extracellular Fluid The extracellular fluid compartment is subdivided in three components ◦ Intravascular = contained within the blood vessels ◦ Interstitial = the solution that exists in the small spaces and gaps between body structures, cells, and tissues ◦ Transcellular = smallest amount of solution; includes mucus, ocular fluid, sweat, secretions of the genitourinary system, cerebral spinal fluid, pleural solution, pericardial fluid, and peritoneal secretions; separated from other fluid by the epithelial lining or other membranes Extracellular Fluid (cont’d) The maintenance of the proportional distribution of the extracellular fluid among these three spaces depends on a variety of factors ◦ Protein content of the blood Albumin pulls fluid toward itself ◦ Integrity of the vascular endothelium ◦ Hydrostatic pressure inside the vessels Tends to force fluid out of the vessels Composition of Body Fluids Solvent = able to hold substances and act to dissolve them ◦ Water is a solvent, and is the main constituent of all body fluids Solute = a substance that is dissolved in the solvent; two major categories ◦ Electrolytes ◦ Nonelectrolytes Solution = the combination of a solvent and a solute Electrolytes Electrolytes Electrolytes comprise 95% of the body’s solute molecules Electrolytes are chemicals that carry an electric charge (ions) Ions converts a solution into a product capable of conducting electricity Anions = ions with a negative charge Cations = ions with a positive charge Electrolytes are expressed in milliequivalents per liter (mEq/L) Electrolytes are crucial to the distribution and movement of water Electrolytes (cont’d) Electrolytes are needed for the maintenance of acid-base balance Electrolytes are needed to carry out cellular reactions Electrolytes are necessary for the transmission of electrochemical impulses in muscles and nerve fibers Major Anions Bicarbonate (HCO3) ◦ Most present in the extracellular fluid at 24mEq/L ◦ Helps in acid-base balance Major Anions (cont’d) Chloride (Cl-) ◦ Most present in the extracellular fluid at 105mEq/L ◦ Aids in fluid balance and osmotic pressure Phosphate (PO4) ◦ Most present in the intracellular fluid at 149mEq/L ◦ Aids in energy storage Sulfate (SO4) ◦ Most present in the intracellular fluid at variable amounts ◦ Assists in protein metabolism Major Cations Sodium (Na+) ◦ Most present in the extracellular fluid at 142mEq/L ◦ Assists with fluid balance and osmotic pressure Calcium (Ca+) ◦ Most present in the intracellular fluid at variable amounts ◦ Responsible for bone growth and assists in blood clotting Magnesium (Mg+) ◦ Most present in the intracellular fluid at 123mEq/L ◦ Assists in enzyme production Major Cations (cont’d) Potassium (K+) ◦ Most present in the intracellular fluid at 100mEq/L ◦ Responsible for neuromuscular excitability ◦ Helps with acid-base balance Fluid and Electrolyte Movement Passive transport = noncarrier-mediated transportation ◦ Movement of solutes through membranes without the expenditure of energy ◦ Types of passive transport – Passive diffusion Facilitated diffusion Filtration Osmosis Active transport = the use of energy to move molecules ◦ Moves substances against the concentration gradient from low to high concentration areas Passive Transport Passive diffusion = the process in which ions, water, and lipid-soluble molecules move randomly in all directions from an area of high concentration to an area of lower concentration through pores in the membrane resulting in even distribution of particles in the fluid ◦ Particles must be small enough to pass through the pores in the membrane Passive Transport (cont’d) Passive diffusion (cont’d) – ◦ If molecules become more populous in one area of the solution compared to another, a concentration difference or concentration gradient results, and the particles will redistribute themselves until they reach a state of equilibrium ◦ An example of this is a metabolic activity that consumes oxygen Causes the diffusion of oxygen from high to lower concentration in the alveoli Reduces the concentration of oxygen in the bloodstream Allows oxygen to be replenished Passive Transport (cont’d) Facilitated diffusion = diffusion across a membrane that is enhanced by a transport protein in the membrane ◦ The transport protein is specific to the substance that is being transported ◦ Glucose is transported in this way Passive Transport (cont’d) Filtration = pressure causes water, ions, and molecules to move from an area of higher pressure to an area of lower pressure ◦ Movement is one-directional ◦ The size of the openings in the membrane determine the size of the particle that can be filtered ◦ Examples include the heart, nephrons in the kidney Passive Transport (cont’d) Osmosis = the passage of water through a semi-permeable membrane in cells and capillaries; water flows from a dilute solution to a more concentrated solution; once the concentration of solutes are equal on each side of the membrane, the flow of water stops and the solutions are isosmotic to each other ◦ Water molecules are very small ◦ A membrane that is semi-permeable is more permeable to water due to its size Passive Transport (cont’d) Osmosis (cont’d) – ◦ An isotonic solution is one in which the salt concentration on either side of the membrane is the same ◦ A hypertonic solution is one in which the salt concentration in the solution is higher, causing water to leave the cell ◦ A hypotonic solution is one in which the salt concentration in the solution is lower, causing water to enter the cell ◦ Osmotic pressure is the amount of hydrostatic pressure needed to draw water across the membrane Passive Transport (cont’d) Osmosis (cont’d) – ◦ A solution with higher osmotic pressure compared to another solution is hypertonic with respect to the other ◦ If one solution has a lower osmotic pressure compared to another solution, it is hypotonic with respect to the other ◦ If two solutions have the same osmotic pressure, they are isotonic with respect to each other Tonicity refers to the osmotic pressure, or tension, of a solution (impacts cell shrinking or swelling) Osmolality Osmolality refers to the concentration of a solute per kilogram of solvent ◦ Measured in weight (kilograms) ◦ Determination of the total number of particles present in blood, urine, or other fluids Osmolality is affected by hydration ◦ Increases with dehydration ◦ Decreases with overhydration Types of osmolality tests ◦ Urine (tests concentrating ability of the kidney) ◦ Plasma (used to test electrolyte imbalances) ◦ Stool (used to diagnose the cause of diarrhea) Osmolarity Osmolarity refers to the concentration of a solute per liter of solution ◦ Measured in volume (liters) and expressed in milliosmols of solute per liter of solution (mOsm/L) Serum osmolarity = 290 – 300 mOsm/L ◦ Refers to the concentration of particles, like sodium, in plasma Estimated serum osmolarity is 2 times the serum sodium level ◦ Sodium is the major solute in plasma ◦ If the sodium level is 145 mEq/L, estimated serum osmolarity would be 290 mOsm/L Osmolarity of IV Solutions Isotonic solutions ◦ 250 – 375 mOsm/L ◦ Have the same osmolarity as normal plasma, so no osmotic pressure difference is created ◦ No fluid movement (fluids stay in the extracellular fluid) ◦ Useful in hemorrhagic conditions because isotonic solutions expand vascular volume quickly and replace extracellular fluid losses ◦ Intracellular and extracellular fluid are isotonic, so red blood cells maintain their concave shape Osmolarity of IV Solutions (cont’d) Isotonic solutions per IV ◦ 0.9% NaCl (Normal saline – NS) Sodium and chloride in water has the same osmolarity as normal plasma No calories or free water (water without solute in it) ◦ Ringer’s solution Contains sodium, potassium, and calcium No dextrose, magnesium, or bicarbonate No calories or free water ◦ Lactated Ringer’s solution (LR) Contains sodium, chloride, potassium, calcium, and lactate in concentration similar to normal plasma No dextrose, magnesium No free water Osmolarity of IV Solutions (cont’d) Hypotonic solutions ◦ <259 mOsm/L ◦ Lower osmolarity than normal plasma ◦ Water moves out of the vessels into the dehydrated cell Decreased vascular volume Increased cell water ◦ Useful in preventing and treating cellular dehydration by providing free water to cells ◦ Never used in acute brain injuries Cerebral cells are very sensitive to free water Absorbed quickly and leads to cerebral edema ◦ Hypotonic extracellular fluid (ion concentration is decreased) causes cells to burst Osmolarity of IV Solutions (cont’d) Hypotonic solutions per IV ◦ 5% dextrose in water (D5W) Isotonic in the bag, but hypotonic in the body Dextrose is rapidly metabolized once infused Leaves free water to shift by osmosis from the vessels into the cells For each liter of D5W, 2/3 enters the cells, and 1/3 remains in the extracellular space ◦ 0.45% saline (1/2 NS) and 0.224% saline (1/4 NS) Provide free water and small amounts of sodium and chloride to the cells Half of each liter moves into the cells, and half remains in the extracellular space Osmolarity of IV Solutions (cont’d) Hypotonic solutions per IV (cont’d) -◦ 5% dextrose in 0.45% saline (D5 ½ NS) and 5% dextrose in 0.225% saline (D5 ¼ NS) Hypertonic in the bag, but hypotonic in the body Composed of hypotonic saline solutions Amount of dextrose does not meet daily nutritional requirements, but is enough to help prevent ketosis and starvation Osmolarity of IV Solutions (cont’d) Hypertonic solutions ◦ ≤ 375 mOsm/L ◦ Higher osmolarity than plasma ◦ Water moves out of the edematous cell into the vessels Increased vascular volume Decreased cell water ◦ Hypertonic extracellular fluid (ion concentration is increased) causes cells to shrink (crenation) Osmolarity of IV Solutions (cont’d) Hypertonic solutions per IV ◦ Carefully controlled to avoid vascular volume overload and cell dehydration ◦ Used to pull excess fluid from the cells and to promote osmotic diuresis ◦ Types of hypertonic IV solutions 3% saline 5% saline 10% dextrose 50% dextrose ◦ IV pump should always be used to control infusion of hypertonic solutions ◦ Frequent monitoring of vital signs, I&O, lung sounds, LOC, and serum sodium levels to avoid hypernatremia and vascular volume overload Active Transport Active transport is necessary to get potassium ions into the cells ◦ Diffusion can not occur because the concentration of potassium is highest in the cells Active transport is necessary anytime there is a concentration differential where a substance must move from lower to higher concentration Adenosine triphosphate (ATP) provides the energy for active transport Active Transport (cont’d) Sodium-potassium pump ◦ Most important pump in the body ◦ Carrier transports sodium out of the cell and pumps potassium into the cell ◦ Maintains higher level of potassium in the intracellular fluid ◦ Essential for neuron and muscle membranes Electrical polarity must be maintained for neurons to generate and conduct electrical impulses Quick Reference for Electrolyte Imbalances Sodium (Na+) helps to balance fluid levels in the body and facilitates neuromuscular functioning Potassium (K+) helps to regulate neuromuscular functioning and osmotic pressure Calcium (Ca+) affects neuromuscular performance and contributes to skeletal growth and blood coagulation Magnesium (Mg+) influences muscle contraction and intracellular activity Chloride (Cl-) regulates blood pressure Hydrogen phosphate (HPO4) impacts metabolism and regulates acid-base balance and calcium levels Bicarbonate (HCO3) assists in the regulation of pH levels in the blood Sodium Sodium is a major cation in the extracellular fluid ◦ Contains 99% of all of the body’s sodium Sodium is responsible for water balance and determination of plasma osmolality ◦ The osmolality of both extracellular fluid and intracellular fluid are isotonic Sodium remains in the extracellular space because it is pumped out of the cells by the sodium-potassium pump The normal range of sodium = 135 – 145 mEq/L Hyponatremia Sodium level <135mEq/L Indicates there is a greater concentration of water than of sodium, which is a hypervolemic state Clients at highest risk for hyponatremia are the very young, the elderly, and women Causes of hyponatremia – ◦ Abnormal loss of GI secretions Vomiting, diarrhea Suction drainage Fistulas Excessive tap water enemas ◦ Excessive sweating ◦ Excessive water consumption ◦ Burns Hyponatremia (cont’d) Causes of hyponatremia (cont’d) – ◦ Disease states that add to increased extracellular fluid volume CHF SIADH Prolonged use of hypotonic intravenous therapy Signs and symptoms (related to the shift of water into the cells) – ◦ Cardiovascular Bounding pulse Tachycardia Hypotension if extracellular fluid volume is decreased Hypertension if extracellular fluid volume is increased ◦ Gastrointestinal Vomiting Diarrhea Hyponatremia (cont’d) Signs and symptoms (cont’d) – ◦ Integumentary Decreased extracellular fluid volume = pale, dry skin; dry mucus membranes Increased extracellular fluid volume = edema, weight gain ◦ Renal Thirst Renal failure ◦ Neuromuscular Weakness Headache Confusion Seizures Nursing interventions – ◦ Assess vital signs Hyponatremia (cont’d) Nursing interventions (cont’d) – ◦ ◦ ◦ ◦ ◦ Diagnostics – ◦ ◦ ◦ ◦ Assess mental status; monitor CNS symptoms Monitor ADH levels Monitor labs, electrolytes Monitor GI losses; perform accurate I&O Obtain daily weight Plasma level <135mEq/L Serum osmolality <270mOsm/kg Serum chloride may be decreased Decreased BUN and hematocrit Treatment – ◦ Intravenous administration of saline solution ◦ Diet therapy Hyponatremia (cont’d) Treatment (cont’d) – ◦ If client with hyponatremia is hypovolemic, treat with NS or LR to correct the extracellular fluid volume deficit ◦ If client with hyponatremia is hypervolemic, administer Mannitol ◦ Replace sodium slowly Too rapid of a correction in sodium further compromises the patient’s condition Hypernatremia may result in brain shrinking Hypernatremia Elderly clients at risk due to age-related decline in thirst sensitivity As sodium increases, water is lost ◦ Water shifts out of cells to establish osmotic equilibrium, causing the brain cells to shrink Brain cells are very sensitive to changes in sodium levels Elevated sodium ↓ Hypertonicity (stimulates thirst and release of ADH) ↓ Body takes in water, which is retained via ADH mechanism Hypernatremia (cont’d) Causes of hypernatremia – ◦ Decreased fluid intake ◦ Increased insensible loss of water Watery diarrhea Osmotic diarrhea (i.e. enteral tube feedings) ◦ High sodium diet ◦ Infusion of sodium-containing fluids ◦ Administration of hypertonic IV solution (i.e. sodium bicarbonate or 3% saline) ◦ Diabetes insipidus Defect in ADH secretion that may cause sodium retention and increased secretion of dilute urine ◦ Head trauma ◦ High glucose levels Osmotic diuresis Hypernatremia (cont’d) Causes of hypernatremia (cont’d) – ◦ Over-the-counter medications with high sodium content Alka-Seltzer Cough syrups Aspirin ◦ Other medications Prednisone Certain antibiotics Signs and symptoms – ◦ Neurological Progressive lethargy, coma Intracranial bleeding due to brain tissue shrinkage ◦ Gastrointestinal Watery diarrhea Nausea Hypernatremia (cont’d) Signs and symptoms (cont’d) – ◦ Cardiovascular Tachycardia Hypertension Decreased cardiac contractility ◦ Integumentary Dry, sticky mucus membranes Rough, dry tongue Flushed skin ◦ Renal Thirst Increased urine output ◦ Neuromuscular Twitching, tremors, seizures, hyperreflexia Agitation, CNS irritability Coma Hypernatremia (cont’d) Diagnostics – ◦ ◦ ◦ ◦ ◦ Sodium plasma level >145mEq/L May see an increase in urine output Chloride level may be elevated Serum osmolality >290mOsm/kg Increased BUN, hematocrit Nursing interventions – ◦ Assess mental status ◦ Monitor for CNS changes ◦ Assess vital signs; assess blood pressure with bounding pulses if client is hypervolemic ◦ Monitor labs ◦ Obtain accurate I&O, daily weight ◦ Maintain client on seizure precautions ◦ Provide a safe environment Hypernatremia (cont’d) Treatment – ◦ Administer 0.9% NS at a rate to correct hypernatremia but avoid cerebral edema Correction usually occurs within 36-72 hours ◦ Once volume deficit is restored, administer fluids with D5W ◦ Administer hypotonic intravenous fluids if there is fluid loss ◦ Administer isotonic IV fluid if there is fluid and sodium loss ◦ Restrict sodium intake ◦ Decrease sodium level slowly as too rapid of a correction may further compromise the patient’s condition Hyponatremia may result in brain swelling Potassium Normal serum potassium level = 3.5 – 5.5 mEq/L Potassium is a major cation in the intracellular fluid Small changes in potassium level have profound effects Potassium’s role in acid-base balance – ◦ In alkalotic states, hydrogen moves out of cells to correct high pH; potassium moves in to cells to maintain an electrically stable state ◦ In acidosis, the reverse of the above actions occurs Potassium (cont’d) Function of potassium ◦ Intracellular Controls cellular metabolism Functions in the regulation of protein/glycogen synthesis ◦ Extracellular Maintains action potential in muscles and neuron cells Assists in controlling cardiac rate and rhythm, conduction of nerve impulses, skeletal muscle contraction, and function of smooth muscle and endocrine tissues The body cannot store potassium ◦ Daily intake of 40 mEq required Potassium (cont’d) The sodium-potassium pump controls the concentration of potassium by removing three 3 sodium ions from the cell for every 2 potassium ions that return to the cell The kidneys eliminate 90% of potassium The remaining potassium is eliminated through stool and perspiration An increased level of aldosterone stimulates and increases excretion of potassium Hypokalemia Serum potassium level <3.5mEq/L Hypokalemia may lead to cardiac and respiratory arrest if not corrected quickly Causes of hypokalemia – ◦ Alkalosis Potassium migrates into the cells as hydrogen ions move out to correct high pH ◦ ◦ ◦ ◦ ◦ Water intoxication (dilutes serum potassium) Potassium-wasting diuretics Excessive loss in GI tract Hemodialysis NPO status without sufficient IV replacement therapy ◦ Malnutrition Hypokalemia (cont’d) Hypokalemia enhances the effects of digoxin ◦ Toxicity may occur at therapeutic levels Signs and symptoms – ◦ Cardiovascular Weak, thready pulse Pedal pulses that are difficult to palpate PVCs Heart block Orthostatic hypotension ECG changes S-T segment depression Flattened T wave Appearance of a U wave ◦ Polyuria Hypokalemia (cont’d) Signs and symptoms (cont’d) – ◦ Respiratory Decreased breath sounds Shallow respirations Dyspnea ◦ Gastrointestinal Abdominal distention Hypoactive bowel sounds Nausea/vomiting Constipation Paralytic ileus ◦ Neurological Anxiety Confusion Lethargy, coma Hypokalemia (cont’d) Signs and symptoms (cont’d) – ◦ Neuromuscular Decreased deep tendon reflexes Muscle weakness/weak hand grasps Leg cramps Nursing interventions – ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Monitor vital signs Monitor serum potassium levels Assess heart rate and rhythm Assess ECG changes Assess respiratory rate, depth, and pattern Protect patient from injury Monitor I&O Monitor for signs of metabolic alkalosis Hypokalemia (cont’d) Nursing interventions (cont’d) – ◦ Administer potassium supplements as ordered ◦ Assess mental status Treated with oral or intravenous potassium replacement ◦ Never administer potassium by IV push or IM as this can lead to fatal arrhythmias ◦ Always use an IV pump for parenteral potassium administration ◦ Observe infusion site frequently for infiltration, phlebitis ◦ Always verify the dosage of potassium in the intravenous solution before hanging ◦ Ensure the diluent is dextrose-free to prevent release of insulin Hypokalemia (cont’d) Treatment (cont’d) – ◦ Do not exceed safe administration rate Potassium infusion per peripheral IV should not be infused more quickly than 20mEq/hour Potassium infusion per central line should not be infused in concentrations greater than 40mEq ◦ If more than 20mEq/hour given, perform continuous ECG monitoring and check serum potassium levels every 4 – 6 hours until normal Hyperkalemia Serum potassium level >5.0mEq/L Potassium moves from the extracellular fluid to the intracellular fluid The myocardium is most sensitive to an increase in potassium levels Changes in the T wave (tall, peaked, or tented) provides the earliest indication that the patient has a high serum potassium level Causes of hyperkalemia – ◦ ◦ ◦ ◦ Rapid infusion of IV potassium Renal failure Adrenal insufficiency Acidosis Hyperkalemia (cont’d) Causes of hyperkalemia (cont’d) – ◦ Addison’s disease Decreased aldosterone leads to sodium depletion and potassium retention ◦ Medications Potassium-sparing diuretics Ace inhibitors ◦ GI bleed ◦ Trauma or ischemia Massive cell damage Burns Signs and symptoms – ◦ Neuromuscular Muscle twitching Paralysis of the arms and legs Hyperkalemia (cont’d) Signs and symptoms (cont’d) – ◦ Cardiovascular Slow, irregular heart rate Decreased blood pressure ECG changes ◦ Gastrointestinal Hypermotility/diarrhea Nausea Abdominal cramping Hyperactive bowel sounds ◦ Respiratory Unaffected until serum potassium level is extremely high Respiratory failure due to muscle weakness Hyperkalemia (cont’d) Nursing interventions – ◦ Monitor potassium levels ◦ Monitor for ECG changes ◦ Monitor I&O Adequate renal function is important for the excretion of potassium ◦ Assess for signs of metabolic acidosis ◦ Monitor ABGs Hyperkalemia frequently seen with acidotic state, though it often resolves when pH is corrected ◦ Monitor labs If dehydration is causing hyperkalemia, hematocrit, hemoglobin, and sodium should be elevated If condition is associated with renal failure, creatinine and BUN levels should be affected Hyperkalemia (cont’d) Treatment – ◦ Discontinue oral and/or IV potassium ◦ Promote potassium excretion Increase urine output Administer potassium-excreting diuretics, like lasix ◦ Administer Kayexalate orally or per rectum Exchanges sodium for potassium in the GI tract and excretes potassium in the stool ◦ Administer insulin and dextrose to shift potassium from the extracellular fluid to the intracellular fluid ◦ Dialysis if hyperkalemia is severe ◦ Administer calcium gluconate IV Does not promote potassium loss, but decreases myocardial irritability Hyperkalemia (cont’d) Treatment (cont’d) – ◦ Administer sodium bicarbonate Makes cells more alkaline, which shifts potassium back into the cells Calcium Calcium is a major cation in the body’s extracellular fluid Calcium is stored in the hard bones Calcium concentration is maintained by the calcium pump, which moves calcium in and out of cells Normal serum calcium levels = 8.5 – 10.5 mg/dL Changes in serum protein (especially albumin) causes changes in calcium level because calcium binds to protein An increase in calcium causes a decrease in phosphorus Calcium (cont’d) Parathyroid hormone ◦ Responsible for the transfer of calcium from bone to plasma ◦ Aids in intestinal absorption ◦ Enhances renal calcium reabsorption Calcium, along with phosphorus, enhances bone strength and durability Calcium helps to maintain cell membrane structure, function, and permeability Calcium affects activation, excitation, and contraction of cardiac and skeletal muscle Calcium helps to activate specific steps in blood coagulation Calcium (cont’d) Calcium assists in the regulation of the acid-base balance Calcium plays a major role in nerve impulse transmission because it determines the speed of ionic refluxes through nerve membranes Hypocalcemia Calcium level < 8.5mg/dL The most common cause of hypocalcemia is inadequate secretion of parathyroid hormone caused by hypoparathyroidism Other causes – ◦ ◦ ◦ ◦ Diarrhea Wound exudate Acute pancreatitis Vitamin D deficiency Signs and symptoms – ◦ Cardiovascular Decreased blood pressure ECG changes with prolonged QT interval Cardiac arrest Hypocalcemia (cont’d) Signs and symptoms (cont’d) – ◦ Respiratory Laryngospasm ◦ Renal failure ◦ Gastrointestinal Hyperactive bowel sounds Diarrhea Intestinal cramps ◦ Musculoskeletal Muscle cramps of the face and/or extremities Bone fractures due to demineralization and/or osteoporosis ◦ Neurological Increased irritability, mental changes Seizures Hypocalcemia (cont’d) Signs and symptoms (cont’d) – ◦ Neuromuscular Paresthesias/numbness and tingling in the hands and feet Hyperactive deep tendon reflexes Tetany Positive Trousseau’s sign Positive Chvostek’s sign ◦ Other signs and symptoms of hypocalcemia Dry, brittle nails Dry hair Bone pain Increased bruising Nursing interventions – ◦ Seizure precautions Hypocalcemia (cont’d) Nursing interventions (cont’d) – ◦ ◦ ◦ ◦ ◦ Assess for Trousseau’s and Chvostek’s signs Assess vital signs, bowel sounds Provide foods high in calcium Monitor calcium labs, ECG Assess for musculoskeletal injury Treatment – ◦ Administer calcium gluconate orally or by IV ◦ Intravenous calcium is 10% calcium gluconate administered by slow IV push Rapid administration can result in bradycardia or cardiac arrest Monitor IV site Monitor for signs and symptoms of hypercalcemia Hypercalcemia Hypercalcemia results from excessive release of calcium from the bones Causes ◦ Metastatic cancer ◦ Hyperparathyroidism ◦ Hyperthyroidism Excessive bone reabsorption ◦ ◦ ◦ ◦ ◦ Thiazide diuretics Excessive calcium intake Immobility Vitamin D intoxication Hypophosphatemia Hypercalcemia (cont’d) Symptoms appear when serum calcium level >12mg/dL Signs and symptoms – ◦ Cardiovascular Hypertension Decreased S-T segments Shortened QT interval Heart block Cardiac arrest ◦ Gastrointestinal Hypoactive bowel sounds Constipation Nausea/vomiting Hypercalcemia (cont’d) Signs and symptoms (cont’d) – ◦ Renal Polyuria Polydipsia Renal calculi ◦ Musculoskeletal Bone fractures/thinning Deep bone pain Decreased muscle tone ◦ Neuromuscular Depressed neuromuscular excitability Decreased deep tendon reflexes Impaired memory Lethargy, confusion, coma Hypercalcemia (cont’d) Nursing interventions – ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Monitor calcium and phosphorus levels ECG monitoring Strict I&O; strain urine Monitor neurological status Assess heart rate and blood pressure Assess vital signs, daily weight Assess level of consciousness Assess bowel sounds Treatment – ◦ Administer 0.9% NS to dilute serum calcium and promote renal excretion Hydration at 3,000 – 4,000 mL/day Hypercalcemia (cont’d) Treatment (cont’d) – ◦ Administer phosphate orally or per enema ◦ Administer loop diuretics (Lasix) to enhance calcium excretion and prevent fluid overload during saline administration ◦ Administer corticosteroids to inhibit calcium absorption in the intestine and increase urinary excretion of calcium ◦ Administer calcium binders ◦ Administer dialysis ◦ Discontinue oral or intravenous calciumcontaining drugs (i.e. antacids) Magnesium Normal magnesium levels = 1.4 – 2.1 mEq/L Uses for magnesium ◦ Enzyme action (needed in at least 300 reactions) ◦ Regulation of neuromuscular activity ◦ Skeletal muscle relaxation following contraction ◦ Powers the sodium/potassium pump ◦ Necessary for maintaining normal heart rhythm ◦ Relaxes the lung muscles responsible for opening the airways Hypomagnesemia Magnesium level <1.4mEq/L The most common cause for hypomagnesemia is alcoholism Other causes – ◦ Altered absorption ◦ Increased renal loss ◦ Prolonged IV therapy without a magnesium supplement due to magnesium moving into the cells ◦ Vomiting ◦ Diarrhea Vitamin D intoxication Hypomagnesemia (cont’d) Signs and symptoms (detectable when levels drop below 1 mEq/L) – ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Muscle twitching, tremors Hyperreactive reflexes Laryngeal stridor Cardiac dysrhythmias Supraventricular tachycardia (SVT) Premature ventricular contractions (PVCs) Ventricular fibrillation Increased susceptibility to digoxin toxicity Mood changes Nausea/vomiting Diarrhea Positive Chvostek’s sign Similar to s/s associated with hypocalcemia Hypomagnesemia (cont’d) Treatment – ◦ Oral replacement of magnesium ◦ Continuous IV infusion of magnesium chloride Rapid infusion may result in respiratory or cardiac arrest, flushing and sweating Diagnostics – ◦ ◦ ◦ ◦ Decreased serum levels of magnesium Increased renal excretion of magnesium Increase in serum calcium Blood gases indicate respiratory or metabolic acidosis Hypermagnesemia Magnesium level >2.1mEq/L The most common cause of hypermagnesemia is renal failure Other causes – ◦ Hyperparathyroidism ◦ Hyperthyroidism ◦ Ingestion of medications high in magnesium Requires emergency treatment ◦ IV calcium gluconate 10% ◦ IV diuretics Hypermagnesemia (cont’d) Signs and symptoms – ◦ ◦ ◦ ◦ ◦ ◦ Flushing Sense of skin warmth Hypoactive deep tendon reflexes Depressed respirations Hypotension Cardiac involvement Bradycardia Heart block Cardiac arrest ◦ Increased susceptibility to digoxin toxicity ◦ Nausea/vomiting ◦ Seizures Phosphorus Normal phosphorus levels = 2.5 – 4.5 mg/dL Uses for phosphorus – ◦ Metabolism of proteins and fats ◦ Formation of adenosine triphosphate ◦ Formation of red blood cell enzymes that aid in oxygen delivery 80% of phosphorus in the body is contained in the bones and teeth 20% of phosphorus is found in the intracellular fluid Phosphorus has an inverse effect on calcium levels (an increase in one will cause a decrease in the other) Hypophosphatemia Serum phosphate level <2.5mg/dL Causes – ◦ Administration of TPN in the absence of a phosphorus malabsorption syndrome ◦ Alcohol withdrawal ◦ Vomiting ◦ Chronic diarrhea ◦ Aluminum-containing antacids ◦ Diuretics ◦ Corticosteroids ◦ Treatment of diabetic ketoacidosis (insulincontaining dextrose causes phosphorus to move into the cells) Hypophosphatemia (cont’d) Signs and symptoms – ◦ Anemia due to increased RBC fragility resulting from low adenosine triphosphate (ATP) levels ◦ Bruising due to platelet dysfunction ◦ Slurred speech ◦ Confusion, coma ◦ Tremors, tetany, seizures ◦ Numbness and tingling of the extremities ◦ Muscle weakness, paresthesias ◦ Chest pain, dysrhythmias due to decreased oxygen availability ◦ Increased rate and depth of breathing due to hypoxemia ◦ Hypoactive bowel sounds ◦ Vomiting Hypophosphatemia (cont’d) Treatment – ◦ Oral phosphate supplements for mild deficiency ◦ IV phosphorus for severe deficiency; watch for hypocalcemia and/or hyperphosphatemia ◦ May be added to TPN ◦ Hypotension may occur if administered too quickly ◦ Watch infusion site for infiltration Hyperphosphatemia Phosphate level >4.5mg/dL Phosphate shifts into the extracellular fluid Primary cause is renal disease Signs and symptoms – ◦ Tetany ◦ Mental changes Sudden hyperphosphatemia (such as in IV administration of phosphates) may result in hypocalcemia Treated by promoting phosphorus excretion ◦ Aluminum-containing antacids bind phosphates in the GI tract Chloride Normal chloride levels in the body = 95 – 108 mEq/L The primary role of chloride is the regulation of serum osmolarity fluid balance Chloride is a major anion in extracellular fluid Chloride has a reciprocal relationship with bicarbonate (HCO3) Chloride binds with other cations (NaCl, HCL, KCL) Chloride (cont’d) Chloride plays an important role in acidbase balance ◦ Chloride shift = an ionic exchange that occurs within the red blood cells ◦ Maintains a 1:20 ratio of carbonic acid and bicarbonate that is essential for pH balance in plasma Hypochloremia Chloride level <95mEq/L Results from vomiting/diarrhea or prolonged use of D5W intravenous solution Signs and symptoms – ◦ Increased muscle excitability ◦ Tetany ◦ Decreased respirations Hyperchloremia Chloride level >106mEq/L Results from severe dehydration or head trauma Signs and symptoms – ◦ ◦ ◦ ◦ ◦ Drowsiness, lethargy Headache Weakness Tremors Cardiac dysrhythmias Acid-Base Balance What is the Acid-Base Balance? In order for homeostasis to be maintained, an equalization must exist between the acidity and alkalinity of body fluids This equalization is known as the acid-base balance The acid-base balance is measured using arterial blood gases (ABGs) The greater the concentration of hydrogen ions, the more acidic a solution becomes ◦ pH is the concentration of hydrogen (H) ◦ The greater the concentration of H, the lower the number of pH Normal ABG Values pH = acid/base 7.35 – 7.45 PO2 = partial pressure of oxygen 80 mmHg – 100 mmHg SaO2 = oxygen saturation 93% – 100% PCO2 = partial pressure of carbon dioxide 35 mmHg – 45 mmHg HCO3 = bicarbonate 22mEq/L – 26mEq/L pH = Potential of Hydrogen The narrow range of pH balance is accomplished by H+ ion balance ◦ HCO3 is regulated by the kidneys ◦ PCO2 is regulated by the lungs pH of body fluids ◦ ◦ ◦ ◦ ◦ ◦ Extracellular fluid = 7.35 - 7.45 Intracellular fluid = 6.9 - 7.2 Urine = 6.0 Gastric secretions = 1.0 - 2.0 Intestinal secretions = 6.6 - 7.6 Bile = 5.0 - 6.0 pH is always the product of two components: respiratory and metabolic Acidosis/Alkalosis Acidosis = pH below 7.35 Alkalosis = pH above 7.45 Respiratory alkalosis = PCO2 <35 Respiratory acidosis = PCO2 >45 Metabolic acidosis = HCO3 <22mEq/L Metabolic alkalosis = HCO3 >26mEq/L Respiratory Acidosis Occurs in response to hypoventilation ◦ ◦ ◦ ◦ ◦ ◦ ◦ Respiratory depression Inadequate chest expansion Airway obstruction Interference with alveolar-capillary exchange COPD Sedative or barbiturate overdose Pneumonia pH decreases while PCO2 increases Respiratory distress Change in level of consciousness Treated by opening airway passages Respiratory Acidosis (cont’d) Nursing assessment – Cardiovascular ◦ Hypotension ◦ ECG shows peaked T waves, prolonged PR intervals, and widened QRS complexes ◦ Peripheral vasodilation with warm, flushed skin ◦ Thready, weak pulse ◦ Tachycardia Respiratory ◦ Dyspnea ◦ Hypoventilation ◦ Hypoxia Respiratory Acidosis (cont’d) Nursing assessment (cont’d) CNS ◦ ◦ ◦ ◦ ◦ ◦ Headache Muscle twitching Seizures Altered mental status Decrease in LOC Drowsiness Diagnostic findings - pH <7.35 PCO2 >45 mmHg Hyperkalemia Respiratory Acidosis (cont’d) Compensation (how the body compensates for respiratory acidosis) – Rate and depth of respirations increased in order to blow off CO2 Kidneys eliminate hydrogen ions and retain bicarbonate HCO3 rises when the body is compensating for acidosis HCO3 increase raises pH Treatment – Treat the underlying cause Improve ventilation (may need a ventilator) Assess respiratory depth and rate Respiratory Acidosis (cont’d) Treatment (cont’d) Pulmonary hygiene ◦ Clear respiratory tract of mucus Provide adequate fluids to liquefy secretions Low flow oxygen for carbon dioxide retention in patients with chronic respiratory acidosis Position patient to facilitate best lung expansion Assess apical pulse, color of skin, nail beds, mucus membranes, LOC Assess for tachycardia or arrhythmias Respiratory Acidosis (cont’d) Treatment (cont’d) Monitor arterial blood gases, potassium Administer medications ◦ Bronchodilators ◦ Antibiotics ◦ Mucomyst to decrease viscosity of pulmonary secretions Oral hygiene Provide a calm atmosphere Keep siderails up and call light within reach Orient patient frequently if needed Respiratory Acidosis (cont’d) Satisfactory Outcomes - ABGs improved to patient’s baseline Decreased anxiety Improved breathing with less effort Freedom from injury No cardiac arrhythmias Improved LOC Normal respiratory rate and depth Respiratory Alkalosis Occurs in response to hyperventilation ◦ Stress ◦ Fever ◦ Pain pH increases while PCO2 decreases Caused by infection, incorrect ventilator settings, respiratory center stimulation as a result of fever, salicylate intoxication Signs and symptoms include headache, dizziness, paresthesias, neuromuscular irritability Respiratory Alkalosis (cont’d) Nursing assessment – Cardiovascular ◦ Increased myocardial irritability ◦ Increased heart rate Respiratory ◦ Rapid, shallow breathing ◦ Chest tightness CNS ◦ ◦ ◦ ◦ Dizziness, light-headedness, blurred vision Difficulty concentrating, anxiety, panic Numbness and tingling in the extremities Hyperactive reflexes, tetany, convulsions Respiratory Alkalosis (cont’d) Diagnostic findings - pH >7.45 PCO2 <35 mmHg Hypokalemia Hypocalcemia Compensation – Kidneys conserve hydrogen and excrete bicarbonate Low HCO3 levels indicates that the body is attempting to compensate Respiratory Alkalosis (cont’d) Treatment – Treat the cause of the condition Assist patient to breathe more slowly Use rebreather mask or paper bag Administer oxygen if patient is hypoxic Administer anxiolytics if needed Provide emotional support Monitor patient’s vital signs Monitor patient’s arterial blood gases Protect patient from injury Respiratory Alkalosis (cont’d) Satisfactory Outcomes - Patient will have a decreased respiratory rate Patient will have absence of numbness or tingling in the extremities Normal (or baseline) ABGs Patient will experience diminished anxiety Patient will be free from injury Metabolic Imbalance Bicarbonate (HCO3) is a direct reflection of the renal system’s ability to compensate for pH changes Normal HCO3 range is 22 – 26 mEq/L HCO3 level <22 indicates acidosis HCO3 level >26 indicates alkalosis Base Excess is an indication of the amount of HCO3 available in the extracellular fluid Normal base excess range is -3.0 to +3.0 A value >3.0 indicates metabolic alkalosis A value <3.0 indicates metabolic acidosis Metabolic Acidosis Causes - Kidney failure Diabetic ketoacidosis Hyperthyroidism Trauma, shock Increased exercise Severe infection, fever Starvation Malnutrition Chronic diarrhea Metabolic Acidosis (cont’d) Nursing assessment – Cardiovascular ◦ ◦ ◦ ◦ Peripheral vasodilation Hypotension Dysrhythmias Cold, clammy skin Respiratory ◦ Deep, rapid breathing (Kussmaul’s respirations) CNS ◦ Drowsiness/lethargy that progresses to coma ◦ Headache, confusion ◦ Weakness Metabolic Acidosis (cont’d) Nursing assessment (cont’d) Gastrointestinal ◦ Nausea/vomiting ◦ Diarrhea ◦ Abdominal pain Diagnostic findings - pH <7.35 HCO3 <22 Hyperkalemia Metabolic Acidosis (cont’d) Compensation (how the body compensates for metabolic acidosis) – Lungs eliminate carbon dioxide Kidneys conserve bicarbonate Treatment – Treat the underlying cause Provide hydration Monitor arterial blood gases Monitor I&O and weight Assess vital signs Assess respiratory rate and depth Metabolic Acidosis (cont’d) Treatment (cont’d) Assess level of consciousness Monitor GI function Administer ECG May need to administer alkalotic IV solution ◦ NaHCO3 ◦ Must be administered cautiously due to possibility of metabolic alkalosis and hypokalemia Metabolic Acidosis (cont’d) Satisfactory Outcomes - Patient will be free from injury Patient will experience no dysrhythmias Normalized ABGs Patient will have no fluid deficits LOC returns to normal Relief of GI symptoms Metabolic Alkalosis Occurs with loss of hydrogen ions ◦ Vomiting ◦ NG suction Occurs with increase in HCO3 due to ingestion of bicarbonate-based antacids Nursing assessment – Cardiovascular ◦ Tachycardia, dysrhythmias ◦ Hypertension Respiratory ◦ Hypoventilation ◦ Respiratory failure Metabolic Alkalosis (cont’d) Nursing assessment (cont’d) CNS ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Dizziness Irritability Tremors Muscle cramps Hyperreflexia Paresthesias of the fingers and toes Tetany Seizures Metabolic Alkalosis (cont’d) Diagnostic findings - pH >7.45 HCO3 >26 Hypokalemia Hypocalcemia (pH increases calcium binding) Compensation – Lungs retain carbon dioxide Kidneys conserve hydrogen and excrete bicarbonate PCO2 increases with compensation Metabolic Alkalosis (cont’d) Treatment – Treat underlying cause Assess level of consciousness Assess vital signs Assess respiratory rate and depth Administer potassium supplement if needed Administer ranitidine or famotidine to decrease secretion of hydrogen from GI drainage Assess I&O Assess arterial blood gases Assess ECG findings Metabolic Alkalosis (cont’d) Satisfactory Outcomes - Hypertension corrected Electrolytes within normal range Normalized ABGs Normal ECG Buffer System Bicarbonate – ◦ Linked to both the respiratory and renal systems ◦ Normal bicarbonate (HCO3) to carbonic acid (H2CO3) ratio is 20:1 ◦ Ratio changes if pH is increased or decreased ◦ Once compensation occurs, the ratio stabilizes Respiratory – ◦ The lungs control the respiratory carbonic acid buffer system, but fatigue quickly ◦ Respiratory rate and depth adjusted in response to carbon dioxide in the extracellular fluid; quick reaction time Buffer System (cont’d) Renal – ◦ The kidneys control the metabolic buffer NaHCO3 (sodium bicarbonate) ◦ Excretes acidic or alkaline urine ◦ Reaction is slow (hours to days) ◦ More effective than the respiratory buffer system Review of Simple Acid-Base Disturbances -- pH <7.35 = acidosis pH >7.45 = alkalosis Abnormal PCO2 = respiratory Abnormal HCO3 = metabolic If the patient is acidotic with a PCO2 >45mmHg, the problem is respiratory If the pt is acidotic with an HCO3 <22mEq/L, the problem is metabolic If the patient is alkalotic with a PCO2 <35mmHg , the problem is respiratory If the patient is alkalotic with an HCO3 >26mEq/L, the problem is metabolic Review of Compensation -If both HCO3 and PCO2 are above or below their normal ranges and are shifting in the same direction, the patient’s buffering system is functioning and trying to bring the acid-base balance back to normal Respiratory Acidosis – ◦ pH <7.35 ◦ PCO2 >45 ◦ HCO3 elevated with compensation (kidneys eliminate hydrogen and retain bicarbonate) Respiratory Alkalosis – ◦ PCO2 <35 ◦ HCO3 decreased with compensation (kidneys conserve hydrogen and excrete bicarbonate)