Tungsten best - elementssph-7-3
advertisement

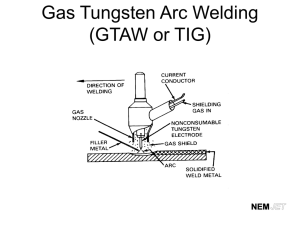
Introduction Tungsten, or Wolfram as it is called in other parts of the world, is a cubic transition metal with colors ranging from steel-gray to tin-white. Discovered by Juan José and Fausto d’Elhuyar, Spanish chemists and brothers, in the year 1783 in Vergara, Spain, tungsten has grown to be mass-produced in countries such as China, Malaysia, Burma, Bolivia, Canada, Australia, Japan and the USA, which are abundant in Name Tungsten or it. Wolfram Element Symbol The Naming of Tungsten The word Tungsten is derived from the Swedish phrase tungs ten, meaning heavy stone. Tungsten’s other name, Wolfram, is derived from the German phrase “wolf rahm” which means wolf dirt, which is derived again from the word “wolframite”, which means devourer of tin, because it was able to “eat through tin. Around the world, different forms of the word tungsten or wolfram are used. Take a look at this table to see tungsten in a variety of languages. Latin Czech Croatian French German Indonesian Chinese Korean Wolframium Wolfram Volfram Tungsténe Wolfram Wolfram 钨 텅스텐 Italian Wolframio Norwegian Wolfram Portuguese Tungstênio Spanish Wolframio Swedish Volfram Malay Wolfram Wu Japanese Teong-seu-ten Greek W (from Wolfram) Atomic Number 74 Atomic Weight 183.85 amu* Melting Point 3695 K (3422°C or 6192°F) Boiling Point 5828 K (5555°C or 6192°F) Element Type Transition Metal Structure Cubic Color Steel-gray to tin-white Density 19.3g/cm3 Period, Group 6,6 *=Atomic Mass Unit = 1.66053886 x 1027 kilograms タングステン Βολφραμιο Ta-n-gu-su-te-n Volframio Discovery and Cultivation Tungsten was originally discovered when the d’Elhuyar brothers did a series of tests on samples of the chemical compound Wolframite ((Fe,Mn)WO4). Today, world-class tungsten industries still make use of the way the d’Elhuyar brothers did. First, Wolframite ((Fe,Mn)WO4) or Scheelite (CaWO4) is crushed, cleaned and treated with alkalis to produce Wolfram-trioxide (WO3). Then, Wolfram-trioxide is heated along with either hydrogen gas or carbon to successfully isolate tungsten metal. Abundance Being quite abundant, tungsten is a major industrial metal. Tungsten is most abundant in the Earth’s crust as compared to seawater and the atmosphere, thus most of the cultivation of Wolframite is taken from the Earth’s Crust. A Picture showing the way electrons are arranged in a Tungsten atom. It also shows the electron shell diagram of a tungsten atom and the electron configuration, 2,8,18,32,12,2. In the Earth’s Crust, tungsten exists at 160.6ppm (parts per million). That can be considered quite abundant as there are other elements which exist only at 50ppm or 60ppm. In the atmosphere, tungsten exists at 411ppm and last but not least (well actually, very least), in seawater, tungsten exists at a minute 9.2 x 10-5ppm. Uses Because of its high melting point, tungsten is used in various places in the electronics industry. Firstly, the everyday light bulb makes use of tungsten as its filament. The filament lights up because the tungsten get hotter and hotter. If the filament were to be something other than tungsten, then the light bulb would easily blow because other metals might not be able to stand the heat created when a certain amount of electricity passes. Secondly, the nozzles of rockets also use tungsten because of the same reason. Tungsten has the highest melting point. If other metals were put as nozzles, the nozzles would burn before even reaching outer space. Thirdly, tungsten is used in tips of drill-bits, high-speed cutting tools and in mining machinery. Again, tungsten is used because of its heat advantage. When the blades of these tools turn very quickly and touch the surface of another object, it becomes really hot and sharp, and thus cuts through the object. If other metals were put in this position, they would have melted or broke after a while during cutting. There are still other uses of tungsten, for its potential is not yet fully harnessed. Perhaps in the near future, tungsten will be used more often. Hazardous Effects Although tungsten can be very useful, as we have discussed, it can also be hazardous. The National Institute for Occupational Safety and Health (United States), which is responsible for developing recommended health and safety standards, said that the maximum exposure to tungsten is 5mg/m3 for people who work with tungsten at an 8 hour per day, 40 hour per week basis. Any amount of exposure greater than this can lead to health problems. Prolonged exposure to high concentrations of tungsten can lead to acute health effects by certain routes of exposure. Irritation to the skin and eyes come from skin and/or eye contact and irritation to the lungs and mucus membrane come from inhalation. On contact, tungsten can make the eyes watery and red and and also the skin red, scaly and itchy. As tungsten can react, always wear protective equipment and follow safe hygiene practices when handling tungsten compounds as they are highly toxic and presents a fire and explosion hazard. Not only does tungsten present a hazard to humans, it can also make animals sick with a few sicknesses such as anorexia, colic, incoordination of movements, trembling, dysprea and weight loss. Thankfully, tungsten has NO effects of hazard on the environment, therefore the environment is not hurt by tungsten or it chemical compounds. Interesting Facts about Tungsten There are lots of interesting facts about tungsten, listed here are just a few. Firstly, tungsten mixed with calcium or magnesium will create phosphors (chemical compounds that glow due to energized particles in them) that are blue and pale blue respectively. Secondly, tungsten mixed with carbon will create tungstencarbide (WC), which can be crated into rings. Thirdly, tungsten may be hard, but when cracked, it becomes brittle and soft to cut through. Lastly, tungsten has the highest melting point among metals and so it is used for a lot of objects or tools that are frequently exposed to high heats. Bibliography Information Barbalace, Kenneth. “Tungsten-W.” Periodic Table of Elements. 22 April 2010 <http://environmentalchemistry.com/yogi/periodic/W.html> Bentor, Yinon. “Periodic Table: Tungsten.” Periodic Table of Elements. 22 April 2010 <http://www.chemicalelements.com/elements/w.html> Gagnon, Steve. “The Element Tungsten.” It’s Elemental: The Periodic Table of Elements. 22 April 2010 <http://education.jlab.org/itselemental/ele074.html> Helmenstine, Anne Marie. “Tungsten or Wolfram Facts.” 22 April 2010 <http://chemistry.about.com/od/elementfacts/a/tungstenwolfram.htm> “Tungsten-W.” Elements – Periodic Table. 22 April 2010 <http://www.lenntech.com/periodic/elements/w.htm> Pictures Robson, Greg. “Electron shell diagram of tungsten.” Tungsten ‘en.wikipedia.org’ 18 Apr 2006 24 Apr 2010 < http://en.wikipedia.org/wiki/File:Electron_shell_074_Tungsten.svg>. “Tungsten.” Tungsten ‘www.chemistry.pomona.edu’ 24 Apr 2010 <http://www.chemistry.pomona.edu/chemistry/periodic_table/Elements/Tungsten/tungsten.ht m>. “Tungsten.” Tungsten ‘students.hthcv.hightechhigh.org’ 24 Apr 2010 <http://students.hthcv.hightechhigh.org/~efregoso/Sophmore/myspaceTemplate.htm>. Arnoldius. “Electric bulb filament.” Filament ‘en.wikipedia.org’ 12 Oct 2005 24 Apr 2010 <http://commons.wikimedia.org/wiki/File:Electric_bulb_filament.jpg>. “Carbide cutting wood saw blade – 002.” Roundsaw ‘www.darchyi.com’ 24 Apr 2010 <http://www.darchyi.com/photo_eng.asp?id=27890>. “8MM Men's Tungsten Carbide Ring with White Carbon Fiber Inaly sizes 8 to 12.” Ring ‘besttitaniumjewelry.blogspot.com’ 24 Apr 2010 <http://besttitaniumjewelry.blogspot.com/2009/09/i-love-ring-and-at-price-you-cant-beat.html>. Sher, Hannah. “Eye Irritation Stock 4.” Eye ‘www.deviantart.com’ 3 Sept 2008 24 Apr 2010 <http://hyannah77-stock.deviantart.com/art/Eye-Irritation-Stock-4-96930569>.