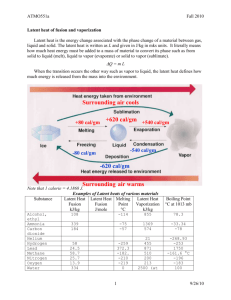
13 14 15 Specific heat capacity and L..
advertisement

1 of 32 © Boardworks Ltd 2006 Do Now •Write down three thing you need to do to work safely in a science laboratory 2 of 32 © Boardworks Ltd 2006 SPECIFIC HEAT CAPACITY Outcomes: • Understand that different materials require different amounts of energy to increase their temperature. • Know the equation. • Be able to use the equation. • Be able to describe an experiment to measure heat capacity. 3 of 32 © Boardworks Ltd 2006 Copy this Glossary absorber – A material that takes in thermal radiation. conduction – The method of heat transfer in solids. conductor – A material that lets heat flow through it. convection – The method of heat transfer in fluids, which occurs because hot fluids are less dense than cold fluids. emitter – A material that gives out thermal radiation. free electrons – Electrons in a metal that are free to move through the metal. heat transfer – The flow of heat energy from a hotter area to a colder area. radiation – Heat energy transferred by infrared waves. This method of heat transfer does not need particles. 4 of 32 © Boardworks Ltd 2006 How does energy affect materials? Do different materials need the same amount of energy to increase their temperature by the same amount? To increase the temperature of 1 kg of water by 1°C, requires 4200 J. To increase the temperature of 1 kg of copper by 1°C, requires 390 J. Water and copper require different amounts of energy because they have different values for a property called specific heat capacity. It is the amount of energy required to increase the temperature of 1 kg of a material by 1°C. So, the specific heat capacity for water is 4200 J/kg°C and for copper is 390 J/kg°C. 5 of 32 © Boardworks Ltd 2006 What is specific heat capacity? The specific heat capacity of a material is the amount of energy required to raise 1 kg of the material by 1 °C. It can be used to work out how much energy is needed to raise the temperature of a material by a certain amount: specific heat temperature energy = mass x x capacity change Energy is measured in joules (J). Mass is measured in kilograms (kg). Temperature change is measured in °C. Specific heat capacity is measured in J/kg/°C. 6 of 32 © Boardworks Ltd 2006 Calculations using S.H.C. Energy absorbed = Mass x Specific Heat capacity x Temp rise J kg J/kg/°C °C The Mr McT equation E = mcΔT 7 of 32 © Boardworks Ltd 2006 For example 0.5 kg of olive oil is heated until its temperature rises by 120 K. If the specific heat capacity of olive oil is 1970 J/kg/°C, how much heat energy was used? Energy absorbed = Mass x Specific Heat capacity x Temp rise Energy absorbed = 0.5 x 1970 x 120 Energy absorbed = 118200 J 8 of 32 © Boardworks Ltd 2006 Practical A 12V V A metal 9 of 32 © Boardworks Ltd 2006 Go through Practical worksheet Follow instructions. Take measurements and do calculations. All students must complete the lab sheet 10 of 32 © Boardworks Ltd 2006 Measuring SHCs • Energy put IN to metal = voltage x current x time • Energy = mass x specific heat capacity x temp rise voltage x current x time = mass x specific heat capacity x temp rise Specific heat capacity = (voltage x current x time)/(mass x temp rise) 11 of 32 © Boardworks Ltd 2006 Specific heat capacity example Using the specific heat capacity of water (4200 J/kg°C), how much energy is needed to increase the temperature of 600 g of water by 80°C in a kettle? Note: mass = 600 g = 0.6 kg specific heat temperature energy = mass x x capacity change energy = 0.6 x 4200 x 80 = 201 600 J 12 of 32 © Boardworks Ltd 2006 Do now Specific heat questions Q5 June 2005 13 of 32 © Boardworks Ltd 2006 Latent heat Learning today • Use the terms latent heat of fusion and give a molecular interpretation of latent heat • Describe an experiment to measure specific latent heats for ice • Test Sunday 1st April 2012 • I will collect your books on Monday Latent Heat Latent heat In a change of state experiment, from the graph you will notice a flat spot the temperature of the salol stopped changing as the salol changed from a liquid to a solid. Temp (°C) Melting point Time (mins) Specific Latent heat “latent” = “hidden” Why does this happen? Specific Latent heat - Copy When a substance changes from solid to liquid or from liquid to gas, it takes energy to change its state. Heat is needed to break the bonds or separate the molecules. The temperature remains the same. When a liquid changes back to a solid energy is released again as the bonds form again. liquid Temp (°C) Melting point solid Time (mins) solid to liquid increasing their potential energy instead of raising the temperature (kinetic energy Specific Latent heat - copy The specific latent heat of a substance tells us how much energy is needed to change the state of 1 kg of substance at constant temperature. Solid to liquid/liquid to solid or liquid to gas/gas to liquid Specific Latent Heat The specific latent heat of fusion (melting) of ice at 0 ºC, for example, is 330000 J.kg-1. This means that to convert 1 kg of ice at 0 ºC to 1 kg of water at 0 ºC, 330000 J of heat must be absorbed by the ice. All at 0°C 1 kg 1 kg 334000 J absorbed Specific Latent Heat Conversely, when 1 kg of water at 0 ºC freezes to give 1 kg of ice at 0 ºC, 330000 J of heat will be released to the surroundings. 1 kg 1 kg 330000 J released All at 0°C Specific Latent Heat -Copy Energy = mass x specific latent heat J kg E = mL J/kg Specific Latent heat L E=mL Do Now question 1 page 119 Learning today • Use the terms latent heat of fusion and fusion and give a molecular interpretation of latent heat • Describe an experiment to measure specific latent heats for ice and steam • Test Sunday 1st April 2012 • I will collect your books Today Specific Latent Heat of Fusion Experiment • • • • • • • Readings required Energy supplied by heater Energy = Power x time Time = Energy = VIt Mass of water E = mL • Latent heat = Energy supplied . mass of water Specific Latent Heat of Fusion Experiment • Energy = Power x time • Time = • Energy = VIt = • Mass of water = • E = mL • L = E /m • Latent heat = Energy supplied . mass of water Task- Evaluation • What are the limitations in our method? • What solutions can you think of? • Why do we wait until the water is dripping? • Does all the heat come from the heater? • Does all the ice remain in the funnel? • Now do Q4 Nov 2005 4 • • • • • Markscheme (a) turn on heater and wait until water starts dripping in beaker empty beaker & replace, start watch stop watch & remove beaker at same time record time find and record mass of water in beaker (b) 60 x t = 120 x 340 t = 680 s (c) (i) ice gains heat from surroundings/ice falls through funnel (ii) lag or fit lid to funnel/place gauze in funnel bottom Specific Latent Heat of Fusion Experiment Latent heat Now do Nov 2005 Q5 Specific Latent Heat of Vaporisation - Copy For water at its normal boiling point of 100 ºC, the latent specific latent heat of vaporisation is 2260000 J/kg. This means that to convert 1 kg of water at 100 ºC to 1 kg of steam at 100 ºC, 2260000 J of heat must be absorbed by the water. vice verse for vapour to liquid All at 100°C 1 kg 1 kg 2260000 J input Specific Latent Heat of Vaporisation Conversely, when 1 kg of steam at 100 ºC condenses to give 1 kg of water at 100 ºC, 2260 kJ of heat will be released to the surroundings. All at 100°C 1 kg 1 kg 2260000 J released Specific Latent Heat of Steam Latent Heat Specific Latent Heat -Copy Energy = mass x specific latent heat J kg E = mL J/kg