Chapter 12 Carbohydrates - Seattle Central College

Chapter 12
Carbohydrates
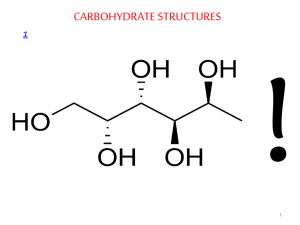
Carbohydrates
• Synthesized by plants using sunlight to convert CO
2 and H
2
O to glucose and O
• Polymers include starch and cellulose.
2
.
• Starch is storage unit for solar energy.
• Most sugars have formula C n
(H
2
O) n
, “hydrate of carbon.”
Chapter 13 2
Classification of Carbohydrates
• Monosaccharides or simple sugars
– polyhydroxyaldehydes or aldoses
– polyhydroxyketones or ketoses
• Disaccharides can be hydrolyzed to two monosaccharides.
• Polysaccharides hydrolyze to many monosaccharide units. E.g., starch and cellulose have > 1000 glucose units.
Chapter 12 3
Monosaccharides
• Classified by:
– aldose or ketose
– number of carbons in chain
– configuration of chiral carbon farthest from the carbonyl group glucose, a
D -aldohexose fructose, a
D -ketohexose
Chapter 12
=>
4
D and L Sugars
• D sugars can be degraded to the dextrorotatory (+) form of glyceraldehyde.
• L sugars can be degraded to the levorotatory
(-) form of glyceraldehyde.
Chapter 12 5
The D Aldose Family
Chapter 23 6
Epimers
Sugars that differ only in their stereochemistry at a single carbon.
Chapter 12 7
Cyclic Structure for Glucose
Glucose cyclic hemiacetal formed by reaction of -CHO with -OH on C5.
Chapter 12
D-glucopyranose
8
Cyclic Structure for Fructose
Cyclic hemiacetal formed by reaction of C=O at C2 with -OH at C5.
Chapter 12
D-fructofuranose
9
Anomers
Chapter 12 10
Mutarotation
Glucose also called dextrose; dextrorotatory.
Chapter 12 11
Epimerization
In base, H on C2 may be removed to form enolate ion. Reprotonation may change the stereochemistry of C2.
Chapter 12 12
Reduction of Simple Sugars
• C=O of aldoses or ketoses can be reduced to C-OH by NaBH
4 or H
2
/Ni.
• Name the sugar alcohol by adding -itol to the root name of the sugar.
• Reduction of
D
-glucose produces
D
-glucitol, commonly called
D
-sorbitol.
• Reduction of
D
-fructose produces a mixture of
D
-glucitol and
D
-mannitol.
Chapter 12 13
Oxidation by Nitric Acid
Nitric acid oxidizes the aldehyde and the terminal alcohol; forms aldaric acid.
Chapter 12 14
Oxidation by Tollens Reagent
• Tollens reagent reacts with aldehyde, but the base promotes enediol rearrangements, so ketoses react too.
• Sugars that give a silver mirror with Tollens are called reducing sugars.
Chapter 12 15
Nonreducing Sugars
• Glycosides are acetals, stable in base, so they do not react with Tollens reagent.
• Disaccharides and polysaccharides are also acetals, nonreducing sugars.
Chapter 23 16
Formation of Glycosides
• React the sugar with alcohol in acid.
• Since the open chain sugar is in equilibrium with its
- and
-hemiacetal, both anomers of the acetal are formed.
• Aglycone is the term used for the group bonded to the anomeric carbon.
Chapter 23 17
Ether Formation
• Sugars are difficult to recrystallize from water because of their high solubility.
• Convert all -OH groups to -OR, using a modified Williamson synthesis, after converting sugar to acetal, stable in base.
Chapter 23 18
Ester Formation
Acetic anhydride with pyridine catalyst converts all the oxygens to acetate esters.
Chapter 12 19
Osazone Formation
Both C1 and C2 react with phenylhydrazine.
Chapter 23 20
Kiliani-Fischer Synthesis
• This process lengthens the aldose chain.
• A mixture of C2 epimers is formed.
Chapter 12 21
Fischer’s Proof
• Emil Fischer determined the configuration around each chiral carbon in
D
-glucose in 1891, using Ruff degradation and oxidation reactions.
• He assumed that the -OH is on the right in the
Fischer projection for
D
-glyceraldehyde.
• This guess turned out to be correct!
Chapter 12 22
Disaccharides
• Three naturally occurring glycosidic linkages:
• 1-4’ link: The anomeric carbon is bonded to oxygen on C4 of second sugar.
• 1-6’ link: The anomeric carbon is bonded to oxygen on C6 of second sugar.
• 1-1’ link: The anomeric carbons of the two sugars are bonded through an oxygen.
Chapter 12 23
Cellobiose
• Two glucose units linked 1-4’.
• Disaccharide of cellulose.
• A mutarotating, reducing sugar.
Chapter 12 24
Maltose
Two glucose units linked 1-4’.
Chapter 12 25
Lactose
• Galactose + glucose linked 1-4’.
• “Milk sugar.”
Chapter 12 26
Sucrose
• Glucose + fructose, linked 1-1’
• Nonreducing sugar
Chapter 12 27
Cellulose
• Polymer of
D
-glucose, found in plants.
• Mammals lack the
-glycosidase enzyme.
Chapter 12 28
Amylose
• Soluble starch, polymer of
D
-glucose.
• Starch-iodide complex, deep blue.
Chapter 12 29
Amylopectin
Branched, insoluble fraction of starch.
Chapter 12 30
Glycogen
• Glucose polymer, similar to amylopectin, but even more highly branched.
• Energy storage in muscle tissue and liver.
• The many branched ends provide a quick means of putting glucose into the blood.
Chapter 12 31
End of Chapter 12
32