Dependence of the FeII/IIIEDTA comlex on pH
advertisement

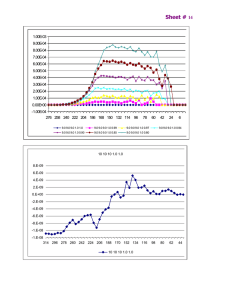
Dependence of the FeII/IIIEDTA complex on pH Ryan Hutcheson and I. Francis Cheng* Department of Chemistry, University of Idaho Moscow, ID 83844 ifcheng@uidaho.edu March 30, 2004 Ryan Hutcheson University of Idaho 1 Importance • First study of the pH dependence of FeII/IIIEDTA • Green chemistry – optimization of O2 activation and pH dependence of the Fenton Reaction • Antioxidants : FeII/IIIEDTA is a good model for low molecular weight biological ligands March 30, 2004 Ryan Hutcheson University of Idaho 2 FeIIIEDTA Speciation Diagram FeIIIEDTA FeIII(OH)2EDTA 1.00E-03 FeIII(OH)EDTA 9.00E-04 8.00E-04 Concentration (M) 7.00E-04 6.00E-04 5.00E-04 4.00E-04 3.00E-04 2.00E-04 FeIIIHEDTA 1.00E-04 0.00E+00 2 3 4 5 6 7 8 9 10 11 pH March 30, 2004 Ryan Hutcheson University of Idaho 3 FeIIEDTA Speciation Diagram FeIIEDTA 1.00E-03 FeII(OH)2EDTA Free Fe+2 FeII(OH)EDTA 9.00E-04 8.00E-04 Concetration (M) 7.00E-04 6.00E-04 5.00E-04 FeIIHEDTA 4.00E-04 3.00E-04 FeIIH2EDTA 2.00E-04 1.00E-04 0.00E+00 2 3 4 5 6 7 8 9 10 11 pH March 30, 2004 Ryan Hutcheson University of Idaho 4 Electrocatalytic (EC’) Mechanism and Cyclic Voltammetry FeIII-L + e- FeII-L FeII-L +H2O2 FeIII-L OH• +OHE: O + ne- = R C’: R + Z = O + Y Regeneration of the FeIIIEDTA within the vicinity of the electrode causes amplification of the CV wave March 30, 2004 Ryan Hutcheson University of Idaho 5 Conditions • All scans – – – – – – – 10mL aqueous sol’n purged w/ N2 for 10-15min 0.1M Buffer - HOAcCl, HOAc, HEPES 5mV/s sweep rate BAS carbon disk electrode BAS Ag/AgCl reference electrode Spectroscopic graphite rod counter electrode BAS CV-50w potentiostat • Cyclic Voltammetric scans of FeIIIEDTA – 1mM FeIIIEDTA • Catalytic scans (Fenton Reaction) – 0.1mM FeIIIEDTA catalytic scans – 20mM H2O2 March 30, 2004 Ryan Hutcheson University of Idaho 6 Cyclic Voltammagrams of II/IIIEDTA Fe 1mM Fe EDTA III -0.000005 0.1M buffer 5mV/s scan rate FeIIIEDTA + e- → FeIIEDTA -0.000004 Current (A) -0.000003 -0.000002 -0.000001 pH 5.5 -0.000005 0 FeIIIEDTA + e- ← FeIIEDTA 0.000001 -0.000004 0.3 0.1 -0.1 0.000002 Current (A) -0.000003 -0.3 -0.5 -0.7 Potential (V) pH 2 -0.000002 -0.000001 pH 11 0 0.000001 0.3 0.000002 March 30, 2004 0.1 -0.1 -0.3 -0.5 -0.7 Potential (V) Ryan Hutcheson University of Idaho 7 E1/2 vs. pH (FeIIIEDTA) FeIII(OH)2EDTA 1.00E-03 0.05 FeIIIEDTA FeIII(OH)EDTA 9.00E-04 0 8.00E-04 7.00E-04 -0.1 6.00E-04 5.00E-04 -0.15 4.00E-04 -0.2 3.00E-04 -0.25 2.00E-04 FeIIIHEDTA -0.3 1.00E-04 0.00E+00 -0.35 2 March 30, 2004 3 4 5 6 7 pH Ryan Hutcheson University of Idaho 8 9 10 11 8 Potential (V) Concentration (M) -0.05 E1/2 E1/2 vs. pH (FeIIEDTA) FeII(OH)2EDTA 1.00E-03 FeIIEDTA Free Fe+2 9.00E-04 0.1 0.05 8.00E-04 0 FeII(OH)EDTA 7.00E-04 -0.05 6.00E-04 -0.1 5.00E-04 -0.15 FeIIHEDTA 4.00E-04 Potential (V) Concetration (M) E1/2 -0.2 3.00E-04 FeIIH2EDTA 2.00E-04 -0.25 1.00E-04 -0.3 0.00E+00 -0.35 2 3 4 5 6 7 8 9 10 11 pH March 30, 2004 Ryan Hutcheson University of Idaho 9 O2 Activation • First example of abiotic RTP oxygen activation able to destructively oxidize organics. • Oxygen activation is pH dependent. Noradoun,C., Industrial and Engineering Chemistry Research, (2003), 42(21), 5024-5030. March 30, 2004 Ryan Hutcheson University of Idaho 10 Reaction Vessel Air flow 2.0 mL 50/50 hexane/ethyl acetate (extraction only) 10.0 mL water 0.44mM EDTA 0.44mM Xenobiotic pH 5.5 – 6.5, unbuffered. Stir bar 0.5g Fe; 20 or 40-70 mesh Noradoun,C., Industrial and Engineering Chemistry Research, (2003), 42(21), 5024-5030. March 30, 2004 Ryan Hutcheson University of Idaho 11 Xenobiotic Oxidation Studies H2O2 + O2 + 2H+ Fe2+ EDTA FeIIEDTA Iron particles 0.1-1 mm FeIIIEDTA + HO- + HO. Aqueous Xenobiotic LMW acids Noradoun,C., Industrial and Engineering Chemistry Research, (2003), 42(21), 5024-5030. March 30, 2004 Ryan Hutcheson University of Idaho 12 Proposed O2 Reduction Mechanism by Van Eldik FeIIEDTAH(H2O) + O2 FeIIEDTAH(O2) + H2O FeIIEDTAH(O2) FeIIIEDTAH(O2-) FeIIIEDTAH(O2-) + FeIIEDTAH(H2O) FeIIIEDTAH(O22-)FeIIIEDTAH + H2O FeIIIEDTAH(O22-)FeIIIEDTAH + H2O + 2H+ 2FeIIIEDTAH(H2O) + H2O2 2FeIIEDTAH(H2O) + H2O2 2FeIIIEDTAH(H2O) + H2O *Proposes H2O2 as intermediate *Saw no evidence of H2O2 Van Eldik, R. Inorg. Chem, 1997, 36, 4115-4120 March 30, 2004 Ryan Hutcheson University of Idaho 13 Van Eldik’s O2 Reduction Van Eldik, R. Inorg. Chem, 1997, 36, 4115-4120 March 30, 2004 Ryan Hutcheson University of Idaho 14 FeIIIEDTA (CN = 7) FeIIEDTA FeIIHEDTA CN = 7 Structures FeIIIHEDTA (CN = 6) O O O O - - - O - N O O - Fe - O HN - Octahedral O N N O Fe O O O O HO O O O O - N - O O N Fe O O HN N O O - - O Monocapped trigonal prismatic (MCP) Pentagonal-bipyramidal (PB) Square Pyramidal O O March 30, 2004 Miyoshi, K., Inor. Chem. Acta., 1995, 230, 119-125. Heinemann, F.W., Inor. Chem. Acta., 2002, 337, 317-327. Ryan Hutcheson University of Idaho 15 Structures cont’d FeIIEDTA FeIIHEDTA pH 3 – pH 4 > pH 4 MCP PB Active site Active site Free Fe+2 < pH 3 Miyoshi, K., Inor. Chem. Acta., 1995, 230, 119-125. March 30, 2004 Ryan Hutcheson University of Idaho 16 Fenton Reaction FeIIIL +e-→ FeIIL E°’=depends on ligand H2O2 + e- → HO• + OHE°=0.32V SHE @pH 7 FeIIL + H2O2 → FeIIIL + HO• + OHOnly iron complexes with E0’ negative of 0.32 V are thermodynamically capable of hydrogen peroxide reduction. However, Fenton inactivity may result from kinetic factors as well. March 30, 2004 Ryan Hutcheson University of Idaho 17 Electrocatalytic CV 0.1mM FeIIIEDTA 20mM H202 0.1M buffer 5mV/s scan rate 0.00007 pH 4 pH 3.5 FeIIIEDTA + e- → FeIIEDTA 0.00006 0.00005 0.00004 0.00003 0.3 0.1 -0.1 -0.3 -0.5 -0.7 Current (A) pH 4 pH 4.5 pH 3 0.00002 0.00001 0.3 0.1 -0.1 -0.3 -0.5 0 -0.7 pH 2.5 pH 2 Potential (V) March 30, 2004 Ryan Hutcheson University of Idaho 18 Fenton Reactivity vs. pH Free Fe+2 1.00E-04 FeIIEDTA 0.00018 9.00E-05 0.00016 8.00E-05 0.00014 7.00E-05 6.00E-05 0.0001 FeIIHEDTA 5.00E-05 0.00008 FeIIH2EDTA 4.00E-05 Current (A) Concentration (M) 0.00012 0.00006 3.00E-05 0.00004 2.00E-05 0.00002 1.00E-05 0.00E+00 0 2 2.5 3 3.5 4 4.5 5 pH Each data point was collected 9 times. March 30, 2004 Ryan Hutcheson University of Idaho 19 Conclusion • E1/2 of the FeII/IIIEDTA complex depends on pH, corresponding to the pH distribution diagram. • Fenton reactivity increases around pH 3.5 due to geometric rearrangement of the FeIIEDTA complex (MCP to PB). March 30, 2004 Ryan Hutcheson University of Idaho 20 Future • pH dependence of Fenton reactivity at higher pH values • Expand van Eldik’s O2 activation to higher pH values March 30, 2004 Ryan Hutcheson University of Idaho 21 Acknowledgments •National Institute of Health •National Science Foundation •University of Idaho •Malcom and Carol Renfrew •Dr. Cheng Group •Dr. Mark Engelmann March 30, 2004 Ryan Hutcheson University of Idaho 22 Nernst Equations E1/2 • pH 2 to pH 3.5 – E1/2(mV) = 83mV – 69.5mV*(pH ) • pH 3.5 to 7 – E1/2(mV) = -89.5mV ± 5.6mV • pH 7 to 9 – E1/2(mV) = 202.8mV – 41.8mV*(pH) • pH 9 to 11 – E1/2(mV) = 409.1mV – 64.6mV*(pH) March 30, 2004 Ryan Hutcheson University of Idaho 23 FeIIIEDTA Model EDTA-4 + H+ → HEDTA-3 HEDTA-3 + H+ → H2EDTA-2 H2EDTA-2 + H+ → H3EDTAH3EDTA- + H+ → H4EDTA H4EDTA + H+ → H5EDTA+ H5EDTA+ + H+ → H6EDTA+2 EDTA-4 + Fe+3 → FeIIIEDTAFeIIIEDTA- + H+ → FeIIIHEDTA FeIIIEDTA- + H20 → FeIII(OH)EDTA-2 + H+ 2FeIII(OH)EDTA-2 → FeIII2(OH)2EDTA2-4 FeIII(OH)EDTA-2 + 2H2O → FeIII(OH)2EDTA-3 + 2H+ H+ + OH- → H2O Fe+3 + OH- → FeIII(OH)+2 Fe+3 + 2OH- → FeIII(OH)2+ Fe+3 + 3OH- → FeIII(OH)3 Fe+3 + 4OH- → FeIII(OH)42Fe+3 + 2OH- → FeIII2(OH)2+4 3Fe+3 + 4OH- → FeIII3(OH)4+8 March 30, 2004 Ryan Hutcheson University of Idaho log β = 9.52 log β = 6.13 log β = 2.69 log β = 2.00 log β = 1.5 log β = 0.0 log β = 25.1 log β = 1.3 log β = 17.71 log β = 38.22 log β = 4.26 log β = 13.76 log β = 11.27 log β = 23.0 log β = 29.77 log β = 34.4 log β = 24.5 log β = 49.7 24 FeIIEDTA Model EDTA-4 + H+ → HEDTA-3 HEDTA-3 + H+ → H2EDTA-2 H2EDTA-2 + H+ → H3EDTAH3EDTA- + H+ → H4EDTA H4EDTA + H+ → H5EDTA+ H5EDTA+ + H+ → H6EDTA+2 EDTA-4 + Fe+2 → FeIIEDTA-2 HEDTA-3 + Fe+2 → FeIIHEDTAH2EDTA-2 + Fe+2 → FeIIH2EDTA FeIIEDTA-2 + OH- → FeII(OH)EDTA-3 FeII(OH)EDTA-3 + OH- → FeII(OH)2EDTA-4 Fe+2 + OH- → FeII(OH)Fe+2 + 2OH- → FeII(OH)2 Fe+2 + 3OH- → FeII(OH)3Fe+2 + 4OH- → FeII(OH)4-2 March 30, 2004 Ryan Hutcheson University of Idaho log β = 9.52 log β = 6.13 log β = 2.69 log β = 2.00 log β = 1.5 log β = 0.0 log β = 14.3 log β = 6.82 log β = 13.41 log β = 18.93 log β = 13.03 log β = 4.2 log β = 7.5 log β = 13 log β = 10 25