vsepr theory - williamsscience
advertisement

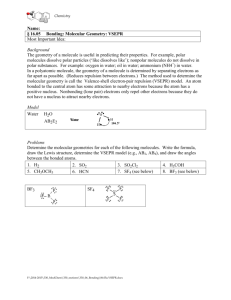
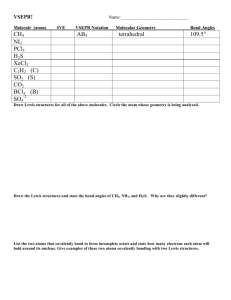
VSEPR THEORY: How do we determine the shapes of molecules and ions? VSEPR THEORY What does VSEPR stand for? Valence Shell Electron Pair Repulsion VSEPR THEORY Why is this important to know? It explains how molecules and ions behave. VSEPR THEORY For example: It explains why water molecules are so good at dissolving ionic substances even though water does not have an ionic bond. VSEPR THEORY: Basic procedure 1) Determine the central atom (usually the atom with the lowest subscript and/or the atom capable of forming the most bonds). Ex: CH4 H2O VSEPR THEORY: Basic procedure 2) Draw the electron dot structure Ex: VSEPR THEORY: Basic procedure 3) Determine the molecular geometry using ALL electron pairs AND atoms around the central atom. VSEPR THEORY: Basic procedure 4) Modify the geometry to determine the molecular shape if non-bonding electron pairs exist Even if the electrons have no atom attached, these unbonded electron pairs still affect the shape of the structure. Molecule Shapes Linear Molecules Linear 2 regions of edensity Bond angle = 180° Angular or Bent Angular or bent At least 3 regions of e- density Only 2 regions bonded Bond angle = 105 ° Triangular Planar (OR Trigonal Planar) Triangular planar 3 regions of edensity All regions bonded Bond angle = 120 ° Tetrahedral Tetrahedral 4 regions of edensity All regions bonded Bond angle =109.5° VSEPR THEORY: Example: SH2 1) Central Atom? S (only 1 atom) VSEPR THEORY: Example: SH2 2) Electron Dot? H S H 2) Bar Diagram? __ H— S —H VSEPR THEORY: Example: SH2 3) Geometry? VSEPR THEORY: Example: SH2 4) Shape? BENT VSEPR THEORY: Example: BF3 1) Central Atom? B (only 1 atom) VSEPR THEORY: Example: BF3 2) Electron Dot? F B F F 2) Bar Diagram? F—B—F F Note that B violates the octet rule— this is an exception! VSEPR THEORY: Example: BF3 3) Geometry? Hint: What is the furthest apart you can spread three atoms attached to a central atom? F F B F VSEPR THEORY: Example: BF3 4) Shape? TRIGONAL PLANAR F F B F VSEPR THEORY: Example: NH3 1) Central Atom? N (only 1 atom) VSEPR THEORY: Example: NH3 2) Electron Dot? H N H H 2) Bar Diagram? H—N—H H VSEPR THEORY: Example: NH3 3) Geometry? Hint: What is the furthest apart you can spread three atoms plus one unbonded pair of electrons attached to a central atom? Think in 3D! ~109.5o N H H H VSEPR THEORY: Example: NH3 4) Shape? Ignore any unbonded pairs of electrons —it IS necessary in this case. trigonal pyramidal ~109.5o N H H H VSEPR THEORY: Example: CH4 1) Central Atom? C (only 1 atom) VSEPR THEORY: Example: CH4 2) Electron Dot? H H C H H 2) Bar Diagram? H H—C—H H VSEPR THEORY: Example: CH4 3) Geometry? Hint: What is the furthest apart you can spread four atoms attached to a central atom? H Think in 3D! C H H H VSEPR THEORY: Example: CH4 4) Shape? Ignore any unbonded pairs of electrons —not necessary in this case. H tetrahedral C H H H VSEPR THEORY: Example: H2O 1) Central Atom? O (only 1 atom) VSEPR THEORY: Example: H2O 2) Electron Dot? O H H 2) Bar Diagram? O—H H VSEPR THEORY: Example: H2O 3) Geometry? ~109.5o O H H VSEPR THEORY: Example: H2O 4) Shape? Ignore any unbonded pairs of electrons —it IS necessary in this case. bent ~109.5o O H H