Powerpoint
advertisement

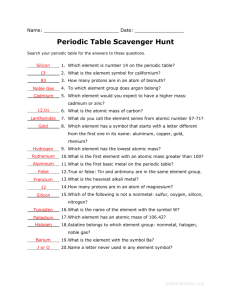
Eleven Gas Elements Chemistry 1 Tenth Grade By: Taylor Welliver Main Menus Nitrogen Neon Oxygen Chlorine Fluorine Argon Quiz Question Krypton Helium Xenon Hydrogen Radon Nitrogen Nitrogen’s atomic number is 7, and it’s atomic weight is 14.0067. Nitrogen's symbol is N and it is located in period 2, group 5 on the periodic table. Nitrogen is a gas at room temperature and is a nonmetal. Oxygen Oxygen’s atomic number is 8 and it’s atomic weight is 15.9994. It’s symbol is O and it’s located in period 2 and group 16 on the periodic table. Oxygen is a gas at room temperature and is a nonmental. Fluorine Fluorine’s atomic number is 9 and it’s atomic weight is 18.99. It’s symbol is F and is it located on the periodic table in period 2 group 17. Fluorine at room temperature is a gas and it is a non-metal. Helium Helium’s atomic number is 2 and it’s atomic weight is 4.003. It’s symbol is He and is it located on the periodic table in period 1 group 18. Helium at room temperature is a gas and it is a non-metal. It’s is also a noble gas. Hydrogen Hydrogen’s atomic number is 1 and it’s atomic weight is 1.008. It’s symbol is H and is it located on the periodic table in period 1 group 1. Hydrogen at room temperature is a gas and it is a non-metal. Neon Neon’s atomic number is 10 and it’s atomic weight is 20.18. It’s symbol is Ne and is it located on the periodic table in period 2 group 18. Neon at room temperature is a gas and it is a nonmetal. Neon is a noble gas. Chlorine • Chlorine’s atomic number is 17 and it’s atomic weight is 35.45. It’s symbol is Cl and is it located on the periodic table in period 3 group 17. Chlorine at room temperature is a gas and it is a non-metal. Argon Argon’s atomic number is 18 and it’s atomic weight is 39.95. It’s symbol is Ar and is it located on the periodic table in period 3 group 18. Argon at room temperature is a gas and it is a non-metal. Argon is a noble gas. Krypton Krypton’s atomic number is 36 and it’s atomic weight is 83.80. It’s symbol is Kr and is it located on the periodic table in period 4 group 18. Krypton at room temperature is a gas and it is a non-metal. Krypton is also a noble gas. Xenon Xenon’s atomic number is 54 and it’s atomic weight is 131.3. It’s symbol is Xe and is it located on the periodic table in 5 period group 18. Xenon at room temperature is a gas and it is a non-metal. Xenon is also a noble gas. Radon Radon’s atomic number is 86 and it’s atomic weight is 222. It’s symbol is Rn and is it located on the periodic table in period 6 group 18. Radon at room temperature is a gas and it is a non-metal. Radon is a noble gas. Quiz Question How many noble gases are there? a) None b) 8 c) 6 d) 2 Sorry try again None is not the correct answer because there are noble gases located in group 18. Sorry try again There is not this many noble gases. Correct! There are six noble gases. Sorry try again There is more then 2 noble gases in group 18. Great Job Please click on the forward button to go back to the home slide for the next student.