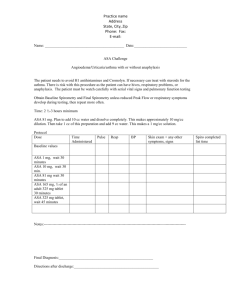
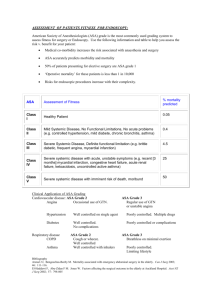
Trial Overview - Clinical Trial Results
Relevant Disclosures for Dr. Mahaffey
•
Research grants:
Significant: Bayer, Boehringer Ingelheim, Bristol-Myers Squibb,
Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson,
Merck, Novartis, Portola Pharmaceuticals, Pozen, Regado,
Sanofi-Aventis, Schering-Plough (now Merck), The Medicines
Company
•
Consultant / advisory board:
Significant: AstraZeneca; Modest: Bayer, Boehringer Ingelheim,
Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Johnson &
Johnson, Merck, Ortho/McNeill, Sanofi-Aventis, Schering-Plough
(now Merck)
Full disclosures at: http://www.dcri.duke.edu/research/coi.jsp
Ticagrelor Compared with Clopidogrel by
Geographic Region in the Platelet Inhibition and Patient Outcomes (PLATO) Trial
Kenneth W. Mahaffey, MD
Daniel M. Wojdyla, MS
Kevin Carroll, MS
Richard C. Becker, MD
Robert F. Storey, MD, DM
Dominick J. Angiolillo, MD, PhD
Claes Held, MD, PhD
Christopher P. Cannon, MD
Stefan James, MD, PhD
Karen S. Pieper, MS
Jay Horrow, MD, MS
Robert A. Harrington, MD
Lars Wallentin, MD, PhD for the PLATO investigators
Background
•
PLATO trial
– 18,624 patients with ACS from 43 countries
– 16% reduction in CV death, MI, stroke (HR 0.84; 95% CI 0.77,
0.92; p<0.001) without increase in overall major bleeding (HR
1.04; 95% CI 0.95, 1.13; p=0.434)
•
31 pre-specified subgroup analyses
•
Treatment by region interaction: p=0.045
•
Less effect of ticagrelor in North America than in the rest of the world (ROW)
•
Exploratory analyses to understand basis of this result
Geographic Regions
CV Death, MI, Stroke
Geographic region
Total patients
KM at month 12
Tic Clop HR (95% CI)
Interaction p-values
Asia /
Australia
1714
Central America /
South America
1237
Europe /
Middle East / Africa
13859
North America 1814
11.4
15.2
8.8
11.9
14.8
17.9
11.0
9.6
0.80 (0.61, 1.04)
0.86 (0.65, 1.13)
0.80 (0.72, 0.90)
1.25 (0.93, 1.67)
0.045
0.01
0.5
1.0
2.0
Ticagrelor better
Clopidogrel better
Methods
•
Independent evaluation by groups at AstraZeneca and the Duke
Clinical Research Institute
•
Investigated systematic errors in trial conduct
•
Evaluated likelihood of the play of chance
•
37 baseline and post-randomization variables identified
•
Cox regression analyses to quantitate the degree to which patient characteristics and concomitant treatments explain the regional interaction
•
Landmark Cox regressions at 8 time points evaluated the association of treatment outcomes with selected factors
No Systematic Errors
•
US and ROW exhibited similar data quality based on query rates
•
Pharmacokinetic substudy samples of US ticagrelor patients had ticagrelor in their plasma
•
US patients had lower study drug compliance
(62% vs. 85%) and higher study drug discontinuation (31% vs. 22%) but similar by treatment group
•
No bias observed in event reporting
Play of Chance
•
Probability of at least 1 false positive treatment interaction (p<0.05) by chance among 31 independent variables is 79%
(adjusted p=0.002)
•
Using overall trial result and distribution of patients and events across regions:
– 32% probability of result favoring clopidogrel in 1 of 4 regions
– 10% probability of result favoring clopidogrel in US and ticagrelor in other 3 regions
•
Distribution of HRs consistent with a common HR of 0.84 across
43 countries:
– HR >1.0: expected in 13 countries; occurred in 12
– HR >1.25: expected in 6 countries; occurred in 3
Baseline Characteristics:
US and Rest of the World
Age, years*
Female sex
Weight, kg*
Hypertension
Diabetes mellitus
Prior MI
Prior PCI
Prior CABG
Smoking: Non-smoker
Ex-smoker
Persistent ST elevation
Troponin positive
US
(n = 1,413)
61 (53 –70)
406 (28.7)
87 (75 –100)
1000 (70.8)
472 (33.4)
387 (27.4)
415 (29.4)
236 (16.7)
416 (29.5)
481 (34.1)
217 (15.4)
1176 (83.2)
Data are n (%) or * median (1st –3rd quartile).
ROW
(n = 17,211)
62 (54 –71)
4882 (28.4)
80 (70 –89)
11,183 (65.0)
4190 (24.4)
3437 (20.0)
2077 (12.1)
870 (5.1)
6840 (39.8)
4195 (24.4)
6791 (39.5)
13,913 (80.8)
Clinical Outcomes
By Region and Treatment
End point Region
Ticagrelor
(N=707 + 8626)
KM (%)
CV death / MI / stroke
CV death
US
ROW
US
ROW
US
12.6
9.6
3.7
4.0
9.6
MI
Stroke
ROW
US
ROW
US
5.5
1.0
1.5
12.2
PLATO major bleeding
All-cause mortality
ROW
US
ROW
10.5
4.2
4.6
Clopidogrel
(N=706 + 8585)
KM (%)
10.1
11.8
2.7
5.3
7.2
6.9
0.6
1.3
11.9
11.1
3.6
6.1
HR (95% CI)
1.27 (0.92, 1.75)
0.81 (0.74, 0.90)
1.26 (0.69, 2.31)
0.77 (0.67, 0.89)
1.38 (0.95, 2.01)
0.80 (0.70, 0.90)
1.75 (0.51, 5.97)
1.15 (0.88, 1.50)
1.05 (0.76, 1.45)
1.04 (0.94, 1.14)
1.17 (0.68, 2.01)
0.77 (0.67, 0.88)
0.0004
0.3730
0.2964
0.7572
0.4696
0.5812
0.0001
P-value
0.1459
<0.0001
0.4468
0.0005
0.0956
KM, Kaplan-Meier; HR, hazard ratio; CI, confidence interval; CV, cardiovascular;
ROW, rest of world; MI, myocardial infarction
High-dose Aspirin Use: Landmark Time Points
ASA: <300 mg is lowdose; ≥300 mg is high-dose.
Effect Modifier Analysis
Entire cohort analysis
Day-4 landmark analysis
Primary Efficacy Outcome
US and Non-US and by ASA Dose
*Hazard ratio not calculated due to small number of events.
Primary Efficacy Outcome
ASA Maintenance Dose
16
14
12
10
8
2
0
6
4
0 60
Tic:
ASA High
Clop:
ASA High
Clop:
ASA Low
Tic:
ASA Low
ASA Low (<300mg):
ASA High (≥300mg):
HR (95% CI), 0.79 (0.71, 0.88)
HR (95% CI), 1.45 (1.01, 2.09)
120 180 240
Days from Randomization
300 360
Landmark Analyses
Primary Efficacy Outcome by ASA Dose
ASA: <300 mg is lowdose; ≥300 mg is high-dose.
Landmark Analyses
Region and ASA
Dose
ASA:
<300 mg is low-dose;
≥300 mg is high-dose.
Limitations
•
Regions were pre-specified subgroups, but analyses for the US alone were not pre-specified
•
The US cohort was relatively small (n=1413), and few patients overall took high-dose maintenance ASA (n=958)
•
Large number of exploratory analyses and no adjustments for multiplicity increases the likelihood of spurious findings
•
Subgroups defined by post-randomization events are potentially biased and hazardous despite rigorous statistical methodology
•
Unknown or unmeasured factors may be contributory
•
No definitive biologic rationale yet explains the ASA finding
Summary
•
In PLATO, ticagrelor reduced CV death, MI, and stroke
•
In a pre-specified analysis of treatment effect across regions, the hazard ratio favored ticagrelor in the rest of the world but not in
North America
•
Statistical analyses by two independent groups identified ASA maintenance dose as a potential explanation of the regional differences
•
The play of chance is another possible explanation
•
These analyses and current ACS treatment guideline recommendations support that, during potent P2Y
12 inhibition with ticagrelor, low maintenance aspirin dose is likely associated with favorable outcomes