What is a Mineral?
advertisement

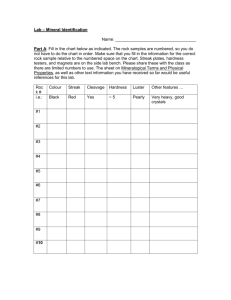
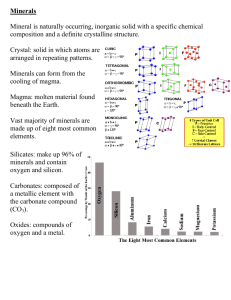
Minerals Minerals Overview atoms minerals rocks History of the Earth Mineral Safety Rules Hand-to-Mouth Contact = use disposable gloves when handling minerals and keep from touching your mouth and your eyes. Wash Hands = after working with minerals wash your hands with soap and water. Samples = must remain in the classroom at all times and returned to the kit when not in use. Poison = most minerals are poisonous, but there is not a large enough sample to cause major harm. DO NOT put the minerals in your mouth! What is a Mineral? Minerals are free uncombined elements or compounds. Their compositions are given as chemical formulas. The formula for fluorite is CaF2. This indicates that the calcium (Ca) atoms have combined with fluorine (F) atoms. The subscripted number (2) shows that there are twice as many fluorine atoms as there are calcium. Minerals are arranged into groups according to the chemical compositions and the crystal structure. Minerals There are four things that distinguish minerals. Naturally occurring Generally inorganic “Constant” chemical composition Crystalline Structure Found in Nature Solid substances found naturally in the Earth. Scientist have learned to make some. Inorganic (usually) Not alive or produced by living process Exceptions graphite (pencil lead) calcite (limestone/marble) aragonite (pearls) “Constant” Chemical Composition Calcite = calcium + carbon + oxygen (CaCO3) Calcite in its purest form is clear, however, the impurities formed by elements change its color – pink, green, gray, etc. Quartz = silicon + oxygen (S1O2) Quartz comes in a variety of colors. Diamonds = carbon (C) Analyzed for color, cut, clarity, and carat. Fancy diamonds such as red, pink, purple, and blue are so rare they are not found on a gemologists color scale. Crystalline Structure The pattern that the atoms arrange in to create an element is the crystalline structure. Halite = sodium + chlorine (NaCl) Halite is regular table salt and has a cube-like structure. Diamond vs. Graphite (C) These two minerals are made of the exact same atoms, but are different because of their crystalline structure. Diamonds vs. Graphite Diamond atoms are strong and close together. Graphite are only strong in one direction and this makes them weak, soft, and flexible. Diamonds can form only at a depth of 120 km or greater within the layers of the earth. Scientists believe that the diamonds have been brought up through kimberlite pipes through some type of explosion and the surface has eroded over millions of years to expose them. Graphite vs. Diamond The Structure of the elements that make up these minerals. Mineral Properties Luster = shiny or dull How well does it reflect light? Light = opaque, translucent, transparent Will the light shine through the mineral? Streak = color of the powdered mineral What is the color of the mineral when powdered? Hardness = soft or hard (MOHS Scale of Harness) How difficult is it to scratch the mineral? Magnetism = magnetic or not Will iron cling to this mineral? Texture = rough or smooth Does it smell like something familiar? Mineral Observations Step-by-Step Procedures Step 1 - Gather materials: hand lens and minerals. Step 2 – Observe the minerals using hand lens and use your senses of touch, smell, and sight. Step 3 – Illustrate the mineral using colored pencils. Step 4 – Using a dropper place a drop of water on the mineral to make it easier to detect smell and to observe the color. Step 5 – Write a sentence describing the texture and smell of the mineral. Mineral Testing Mineral A (illustration) Luster Hardness Light Streak Mineral Testing Mineral A (illustration) Luster Dull Hardness (Mohs Scale) Soft (6.0 ) Light opaque Streak white Streak Color Streak Color Test Light Light Test Luster Luster Test Mohs Scale of Hardness Way back in 1822, the German mineralogist, Friedrich Mohs developed the 10 point hardness scale that is still used today. Hardness Mohs Scale of Hardness Fredrick Mohs developed this scale to categorize minerals. Any mineral with a higher number will scratch a mineral of a lower number. 1 Talc Talcum powder. 2 Gypsum Drywall and Plaster of Paris. Fingernail (2.5) 3 Calcite Copper Penny. 4 Fluorite Used to lower the melting temperature of iron. 5 Apatite Window Glass, is found in teeth and bones (5.5) 6 Orthoclase Steel File also used in ceramics (6.5), 7 Quartz Used in electronics and glass 8 Topaz Sandpaper, emeralds and aquamarines are varieties of beryl with a hardness of 8. 9 Corundum Sapphire and Ruby are types of corundum. They are two to five times as hard as topaz. Used to grind things down. 10 Diamond Four times as hard as corundum. Hardness Test Power Conclusion 1. Refers back to the prediction. I thought that . . . 2. Answers the question. I found out . . . 3. Future Research. In the future I would like to . . . Glossary Color Streak: the color that a mineral leaves behind when you rub it on a black or white tile. Light: A mineral’s light transmissivity can be described as opaque, transparent, or translucent. Luster: describes the way the surface of the mineral reflects light. Mineral: a solid material with atoms arranged in a repeating pattern. Mohs Scale of Hardness: A scale of 10 minerals used to compare all minerals from softest to hardest. Glossary Opaque: When light is not able to travel through a solid surface . Rock: Material made up of one or more minerals. translucent: When some light can travel through a solid object. transmissivity: The ability for light to travel through a solid object. transparent: When all the light can travel through a solid object. (clear)