Minerals PPT
advertisement

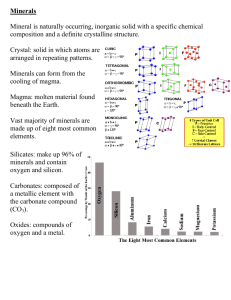
Defining the Atom I. Atomic Theory A. B. C. D. E. F. All matter is made up of very tiny particles called atoms Atoms of the SAME element are chemically alike The atoms of an element have a definite mass that is characteristic of the element The Atoms of different elements have a different number of protons in their nucleus (so atoms of different elements have different atomic masses). Atoms are not subdivided, created, or destroyed in chemical reactions Sizes of Atoms: Atomic Radius 1. Atoms are about 1 X 10-12 m in size…so cannot be seen (why we call it the atomic “theory”) What makes elements different? II. What is an element? 1. The atoms of different elements have a different number of protons in their nucleus 2. An element is a substance that cannot be broken down to any simpler substance; made up of all the same atoms each with the same number of protons in the nucleus 3. Examples: Hydrogen has just one proton in it’s nucleus, Lithium has 3; Berillium has 4; Sodium has 11. 4. The number of protons in the nucleus is called the atomic number! Structure of the Atom III. Structure of the Atom: 1. 2. All atoms are composed of subatomic particles called electrons, protons, and neutrons Electrons, protons, and neutrons are different in terms of their mass, electrical charge, and location in the atom IV. Protons and neutrons have the same mass, while electrons are much lighter. V. Protons have a positive charge, neutrons are neutral (no charge) and electrons have a negative charge VI. Protons and neutrons are found in the center of an atom (nucleus), while electrons are attracted to the protons on the outside of the nucleus (opposite charges attract) I. Minerals • All minerals are made up of single elements or compounds. • Element = a substance that cannot be broken down to any simpler substance; made up of atoms • Atom = smallest part of an element; has all the properties the element has but cannot be seen even with tools • Compound = substances consisting of more than one element Minerals - definition 1. Naturally Occurring synthetic substances are not minerals 2. Inorganic plant and animal activities (coal, pearls are not minerals) 3. Solids 4. Definite Chemical Composition 5. Crystalline Structure a crystal is a regular geometric solid shape; reflects the ordered internal arrangement of atoms 6. Definite Set of Physical Properties Physical Properties of Minerals • • • • • • • Can be used to identify minerals! Color Luster Streak Odor Hardness Breakage Pattern – Cleavage – Fracture Color • Very useful property only for some minerals • Not useful for other minerals because: – 1. Many minerals come in multiple colors – 2. One color (such as white) can be seen in many minerals – May be used for some of the obvious minerals that are almost always one color (sulfur = yellow) These are all samples of the same mineral! Quartz comes in many colors! These four minerals (and many others) are all white! Is color always a useful physical property for identifying minerals? •NO! • But it sure makes looking at minerals a lot more interesting! Luster Luster... • The way light shines or glares off the surface of minerals • Better than color to identify minerals • Many types of luster have been described • Somewhat subjective • Need to familiarize yourself with terms... Luster... • Metallic – looks like metal in the way the mineral reflects light (Galena or Pyrite) • Non-Metallic – Does not look like polished metal, so other terms are used. Types of Non-Metallic Luster • • • • Vitreous - glassy, like glass (quartz) Resinous - a dull shine, like a resin (amber, olivine) Waxy - dull shine like candle wax (sulfur, olivine) Adamantine - brilliant glow, beam of light at a certain angle (diamond) • Earthy - no shine at all (bauxite) • Pearly - looks like pearls when light is reflected off the minerals surface (opal, garnet) The way light reflects off the surface of a mineral (luster) is useful in identifying the mineral Galena definitely has a metallic luster as it looks like polished metal Graphite may display a “sub-metallic” luster, as it looks like dull metal Quartz has a glassy look, which is called a “vitreous” luster Match the luster description with the appropriate luster term by writing the correct number next to the term: Luster Term Definitions _______Pearly 1. a brilliant glow at certain angles under the light; such as diamond _______Dull (Earthy) 2. shines like reflected glass _______Vitreous 3. no shine at all even under bright light _______Waxy 4. glistens like pearls under light _______Metallic 5. shines like candle wax, a dull glow _______Adamantine 6. gives off a glare like a gold necklace or silver bracelet Streak • The color of the powder of a mineral obtained by rubbing the sample across an unglazed porcelain plate (called a streak plate) • Most minerals display only one color of streak • Examples: – Hematite always has a reddish brown streak, no matter what color the sample is – Sulfur has a yellow streak – Sphalerite has a yellow streak – Magnetite has a black streak When checking the streak of a mineral, be sure to follow these steps: • Hold streak plate carefully with index finger and thumb of one hand • Hold streak plate firmly against desk top • Rub mineral across plate firmly to powder it Checking streak... • Be careful not to break off the corners of the streak plate. • Some streak plates have sharp edges so WATCH OUT! • Check the color of the minerals powder to determine streak. Color, Luster, and Streak Quiz! • Color is the most useful property used to identify a mineral. • FALSE! Too many minerals are the same color and many are white, green, black, etc. Color, Luster, and Streak Quiz! • Vitreous is a type of non-metallic luster seen on minerals that reflect light as glass does. • True! Vitreous is glassy. Color, Luster, and Streak Quiz! • The way a mineral reflects light is called streak. • FALSE! Luster is the way a mineral reflects light. Streak is the color of the mineral in powder form. Color, Luster, and Streak Quiz! • Determining the streak of a mineral is done by whacking the mineral with a sledge hammer until it is pulverized. • FALSE! Streak is easily determined by rubbing the mineral across an unglazed porcelain plate. Color, Luster, and Streak Quiz! • The streak of a mineral will always be the same as the mineral color. • FALSE! Streak is particularly useful when it is different than the color of the mineral itself. Hardness of Minerals • Hardness is the resistance of a mineral to being scratched • All minerals are assigned a hardness value from 1 – 10 based upon “Moh’s Hardness Scale” • Hardness is one of the most useful properties because: – Resistance of a mineral to being scratched never changes among different samples of the same mineral – Hardness is easily determined using your fingernail, a glass plate, a steel nail, and reference minerals Hardness = the resistance of a mineral to being scratched • Mineral Name (Moh’s Scale) – 1. Talc – 2. Gypsum * 2.5 Human Fingernail – 3. Calcite – 4. Fluorite – 5. Apatite * 5.5 – – – – – Typical Uses body powder building (wallboard, etc.) lenses in microscopes toothpaste fertilizer GLASS PLATE 6. Feldspar 7. Quartz 8. Topaz 9. Corundum 10. Diamond floor tiles (mall floors) watches, abrasives, etc. gemstones abrasives, cutting tools saws, “a girl’s best friend” A visual look at Moh’s Hardness Scale Diamonds are so prized because they are harder than any other substance, so they are extremely resistant! Hope Diamond Diamond particles that are not used as gemstones can be used in cutting saws, and are able to cut all other industrial materials. Hardness - continued… • Hardness is a very useful property, since a mineral’s hardness is constant – it is the main way we can separate “soft” minerals (1-5 on Moh’s scale) from “hard” minerals (6-10) • “Hard” minerals such as quartz are used as abrasives • Fingernail is a 2.5 on Moh’s scale, so can be used to identify very soft minerals (scratched by fingernail) • Steel nail is about 5.0 on Moh’s scale – (softer than glass) Hardness... • How do we determine the hardness of a mineral? – First, we rub an edge of the mineral across a glass plate, to see if it scratches the glass. Remember, a glass plate is about 5.5 on Moh’s hardness scale. – Once we determine whether the mineral is harder (scratches) or softer (does not scratch) than 5.5, we use the reference minerals of Moh’s hardness scale and other items such as our fingernail, steel nail, etc. to more accurately determine hardness. Checking for Hardness... • Be sure to hold the glass plate down firmly on the desk • Be careful of sharp edges on the glass plate! • Glass can be very fragile and sharp! • Wipe mineral across glass plate to see if it scratches the glass Checking hardness... • Check to see if the mineral actually made a groove in the glass or just merely rubbed off on the glass • A mineral is harder than glass if there is a definite groove or scratch in the glass plate. Hardness of Minerals… • If the mineral does not scratch glass (less than 5.5), try to scratch it with your fingernail – If your fingernail can scratch the mineral, it is also less than 2.5 on Moh’s scale – If your fingernail does not scratch the mineral, it is between 2.5 and 5.0 on Moh’s scale. • KNOWING THE HARDNESS VALUE WILL MAKE IDENTIFYING THE MINERAL MUCH EASIER!!!! Hardness... Procedure for Determining a Minerals Hardness Scratches Glass Harder Than 5.5 Check against a piece of feldspar, then quartz then topaz then corundum Does Not Scratch Glass Softer Than 5.5 Fingernail scratches mineral Softer than 2.5 Check against talc then gypsum Fingernail does not scratch mineral Check against calcite then fluorite, apatite Breakage Pattern • 1. Cleavage - the tendency of a mineral to split consistently along certain planes of weakness • 2. Fracture - no definite planes of weakness so mineral just breaks along irregular surfaces Cleavage... • Minerals may show one or more planes of weakness: – – – – one plane of weakness = basal (muscovite mica) two planes of cleavage at 90º (orthoclase) three planes of weakness at 90º = cubic (halite) three planes not at 90 º = rhombohedral (calcite) • You must look carefully for “flat” surfaces which give off a glare to find the cleavage in a mineral • Not always obvious Cleavage... • Some minerals such as mica break apart along one smooth flat plane, called BASAL cleavage. • This sample has also been cut into straight edges. Cleavage... Basal cleavage (muscovite mica) 2 directions of cleavage at 90º (feldspar) Cubic cleavage (galena) Fracture... • Conchoidal fracture - irregular breakage surfaces are somewhat rounded (like the way glass breaks) – Quartz is noted for its conchoidal fracture • Hackly fracture - breakage surfaces are jagged (such as the mineral copper) • Uneven fracture - some minerals break apart in no distinguishable pattern. Fracture... Hackly fracture (copper) Conchoidal fracture (Quartz) Quiz… • Minerals are mostly man-made. • False. Minerals are naturally-occurring. Quiz... • Minerals usually exhibit a characteristic streak, or powder color. • True. Quiz... • Moh’s hardness scale is used to determine a mineral’s luster. • False! Moh’s scale is used to determine hardness. Quiz... • Minerals contain every element on the periodic table. • False. Minerals are composed of one or several of the elements, in a specific chemical formula. Quiz... • If a mineral shines like metal when reflecting light it has a metallic luster. • True. Quiz... • The physical properties of minerals are used to distinguish one mineral from another. • True. Quiz... • Cleavage is the breaking of a mineral along irregular surfaces. • False. Cleavage indicates certain planes of weakness within the mineral which causes the mineral to always break along those planes. Quiz... • When checking for the hardness of a mineral, you should always check to see if it scratches diamond first. • False. See if the mineral scratches glass first, then use your fingernail or reference minerals to more accurately determine hardness. Quiz... • When checking the streak of a mineral, always hold the streak plate flat on the desk and rub the mineral across the plate to powder it. • True Quiz... • If a mineral appears like glass when reflecting light it is said to have an earthy luster. • False. Vitreous is the term used to describe glassy luster. Families of Minerals • Minerals can be categorized into groups (families) based upon their composition and physical properties. • Some minerals consist of only one element, and are called “Native Minerals”. – Examples: Gold (Au), Copper (Cu) Sulfur (S) and Diamond (C) are all Native Minerals Examples of native minerals: • • • • • Gold (Au) Silver (Ag) Copper (Cu) Sulfur (S) Graphite or Diamond (C) • Platinum (Pt) Native Minerals 23. Most native minerals have monetary value. – This means that they are very valuable and are the most sought after minerals. 24. Most metals are considered native minerals and usually have multiple uses. 25. Native minerals are known for special physical properties. – conductivity (electricity) – pliability (can be bent into shape) – durability (very strong) Non-Native Minerals (Compounds) 26. Most minerals are made up of two or more elements combined (called compounds). • The following minerals are all compounds! • • • • • • Orthoclase (KAlSi3O8) Gypsum (CaSO4·2H2O) Quartz (SiO2) Halite (NaCl) Calcite (CaCO3) Pyrite (FeS2) *****The most common minerals are made of the most abundant elements in Earth’s crust. Eight most abundant elements in the Earth’s crust: ELEMENT (% BY MASS) 1. Oxygen (46.10%) only 2. Silicon (28.20%) silly 3. Aluminum (8.23%) artists 4. Iron (5.63%) in 5. Calcium (4.15%) college 6. Sodium (2.36%) study 7. Potassium (2.09%) past 8. Magnesium (2.33%) midnight Families of Minerals 27. Most minerals are combinations of the most abundant elements found in Earth’s crust. 28. Oxygen and Silicon combine readily with each other and with other elements to form a family of minerals called the Silicates. 29. The silicates are the most common family of minerals and make up over 90% of all minerals – This is because Oxygen and Silicon are the most common elements in Earth’s crust! Which minerals are silicates? • • • • • • • • • Orthoclase (KAlSi3O8) Gypsum (CaSO4·2H2O) Quartz (SiO2) Halite (NaCl) Calcite (CaCO3) Pyrite (FeS2) Augite (Mg,FeSiO3) Olivine (Mg,Fe)2SiO4 Biotite K(Mg,Fe)3AlSi3O10(OH)2 Special Properties of Minerals: 30. Magnetism - the ability to attract a magnet (magnetite, powder of Ilmenite) 31. Effervescence (reaction with acid) - bubbly reaction in HCl, “fizzing” (calcite, powder of dolomite) 32. Specific Gravity (Density) - some minerals have atoms very tightly packed, and feel heavier because they are more dense! Galena is very dense, calcite is not 33. Specific Gravity - definition Specific Gravity = How many times heavier a mineral is than an equal volume of water. This depends on it’s density. • Formula for Specific Gravity S.G. = weight of mineral in air (g) (weight in air) - (weight in water) • Specific gravity answers have no units • Specific gravity is basically how much more dense a mineral is than an equal volume of water Special Properties of Minerals: 34. Labradorescence - the colorful hue seen at certain angles while the mineral labradorite reflects light; looks blue-purple 35. Striations - a series of very fine parallel grooves found on the cleavage surfaces of some minerals (Plagioclase Feldspars have striations, other feldspars don’t: ex.: albite, labradorite have striations) 36. Double-refraction - the doubling of images when light passes through mineral (calcite) Special Properties of Minerals... 37. Taste - Halite tastes like salt (it is salt) – DO NOT TRY THIS IN LAB! 38. Odor - Sulfur smells like rotten eggs – Sometimes the powder of a mineral (streak) has a certain odor (sphalerite smells like sulfur when streaked) 39. Fluorescence - some minerals give off a specific color when viewed with an ultraviolet light! 40. Piezoelectricity - emits an electric current when put under pressure (quartz) 41. Without minerals, life would be very different! Minerals have many uses... • Jade altar bowl shown here • Pottery, jewelry, industry, electricity • Quartz alone is used as: – – – – an abrasive in watches (piezoelectricity) in telephones many other uses • Think about all of the minerals we use in the classroom! • Think of how many minerals it takes to make an IPOD or cell phone! Mineral Quiz: (true or false) • All minerals have the same chemical composition? • False. Each mineral has its own ingredients which can be determined in a special laboratory. Quiz cont... • Minerals occur in only one color? • False. Some minerals such as calcite and quartz come in many different colors. Quiz cont... • A mineral must be a solid (no liquids or gases). • True. Quiz cont... • Hardness, luster, and streak are physical properties that are useful in identifying minerals. • True. Quiz cont... • Talc is the softest mineral on Moh’s hardness scale while diamond is the hardest. • True. Quiz cont... • Hardness is determined by scratching a mineral across an unglazed porcelain plate. • False. Hardness is checked by scratching the mineral against a glass plate and then against reference minerals. Quiz cont... • Cleavage is the ability of a mineral to break along atomic planes of weakness. • True. Quiz cont... • About 8 elements make up most of the minerals found on Earth. • True. Quiz cont... • All minerals exhibit some form of cleavage. • False. Some minerals display no special planes of weakness and exhibit fracture. Quiz continued... • The basic building block of the silicate family of minerals is called the silicon-oxygen tetrahedron. • TRUE! Quiz cont... • There are six components to the definition of a mineral: – Naturally Occurring – Inorganic – Solids – Definite Chemical Composition – Crystalline Structure – Definite Set of Physical Properties • TRUE! How Are Minerals Formed? • Minerals are formed by the process of crystallization as a result of: 1. Cooling and solidification from magma (liquid rock) (ex: olivine, plagioclase feldspar, potassium feldspar) 2. Precipitation from water caused by evaporation, or over- saturation of minerals (ex: halite & calcite) 3. Chemical reactions from pressure and temperature changes within the Earth (ex: talc, muscovite mica) 4. Chemical reactions from hydrothermal solutions (hot water & dissolved ions from 100°C to 300°C) (ex: bornite, chalcopyrite) How Are Minerals Formed? • Minerals are formed by the process of crystallization as a result of: – cooling and solidification from magma (liquid rock) – precipitation from water caused by evaporation, chemical reactions, and temperature changes – rearrangement of atoms in existing minerals subjected to conditions of high temperatures and pressures found deep within the earth Silicon-Oxygen Tetrahedron... • The tetrahedron is made up of four oxygen atoms packed closely around a silicon atom • The tetrahedron continues outward in all directions like legos, and combines with other metal elements to build the different minerals of the silicate family. • All of the first 18 minerals in your kits are silicates, except for calcite, gypsum, and halite. Silicon-Oxygen Tetrahedron... • This is a model of the silicon-oxygen tetrahedron • The white balls represent oxygen atoms • The black ball in the center represents the silicon atom. • Imagine millions of these linked together in 3D Silicon-Oxygen Tetrahedron... • There are many models used to show the silicon-oxygen tetrahedron. • The way the tetrahedrons are linked and the other elements it binds with will determine all the physical properties of silicate minerals Silicon-Oxygen Tetrahedron... • Silicates include the minerals quartz, feldspar, augite, and hornblende. • The very different physical properties these minerals exhibit is due to the slightly different chemical formulas and how the silicon-oxygen tetrahedron is linked!