Bonding-Nam_writ- transit
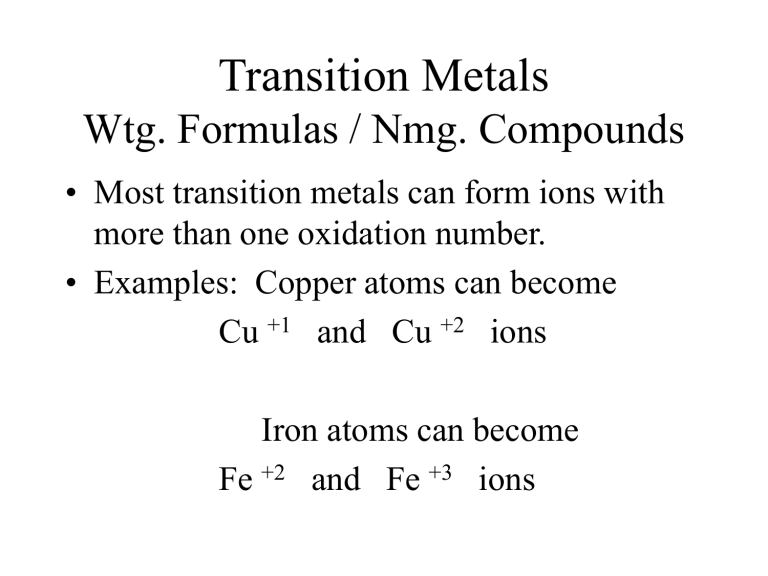
Transition Metals
Wtg. Formulas / Nmg. Compounds
• Most transition metals can form ions with more than one oxidation number.
• Examples: Copper atoms can become
Cu +1 and Cu +2 ions
Iron atoms can become
Fe +2 and Fe +3 ions
Writing Formulas w/Transition Metals
Iron (III) Oxide
• Write the symbol for the transition metal.
Ex. Fe
• Take the oxidation number of the metal, written in parentheses, and write it as the oxidation number.
Ex. Fe +3
Writing Formulas w/Transition Metals
Iron (III) Oxide
• Write the symbol for the nonmetal.
Ex. O
• Look up its oxidation number on the periodic table, and add it to the symbol.
Ex.
O -2
Writing Formulas w/Transition Metals
Fe
+3
O
–2
• Find the common factor between the two oxidation numbers. In this case = 6
• Decide how many of each ion is needed to make the charge equal to the common factor. In this case 2 Fe and 3 O ions.
• Use this number of ions as the subscript for the element, and write the formula.
Fe
2
O
3
Copper (I) Sulfide
• Write the symbol for the transition metal.
Ex. Cu
• Take the oxidation number of the metal, written in parentheses, and write it as the oxidation number.
Ex. Cu +1
Copper (I) Sulfide
• Write the symbol for the nonmetal.
Ex. S
• Look up its oxidation number on the periodic table, and add it to the symbol.
Ex.
S -2
Copper (I) Sulfide
Cu
+1
S
–2
• Find the common factor between the two oxidation numbers. In this case = 2
• Decide how many of each ion is needed to make the charge equal to the common factor. In this case 2 Cu and 1 S ion.
• Use this number of ions as the subscript for the element, and write the formula.
Cu
2
S
Naming Compounds w/Transition Metals
FeO
• Look up the nonmetal on the periodic table.
Find its oxidation number.
Oxygen O -2
• Look up the metal on your ion chart. Find the possible oxidation numbers.
Fe +2 or Fe +3
Fe
+2
or Fe
+3
O
-2
• Decide which ion will form in the proper ratio with the known charge on the oxygen ion.
FeO
• Iron bonds in a 1 to 1 ratio with oxygen, therefore, the iron ion must have a +2 charge.
• Name the compound, indicating the oxidation number of the metal in parenthesis.
Iron (II) Oxide
Fe
2
O
3
• Look up the nonmetal on the periodic table.
Find its oxidation number.
Oxygen O -2
• Look up the metal on your ion chart. Find the possible oxidation numbers.
Fe +2 or Fe +3
Fe
2
O
3
• Decide which ion will form in the proper ratio with the known charge on the oxygen ion.
Fe +2 or Fe +3
• Iron bonds in a 2 to 3 ratio with oxygen. Three oxygen atoms will have a charge of -6. Therefore, two iron ions must equal +6. It must be Fe +3.
• Name the compound, indicating the oxidation number of the metal in parenthesis.







