Experiment 21
advertisement
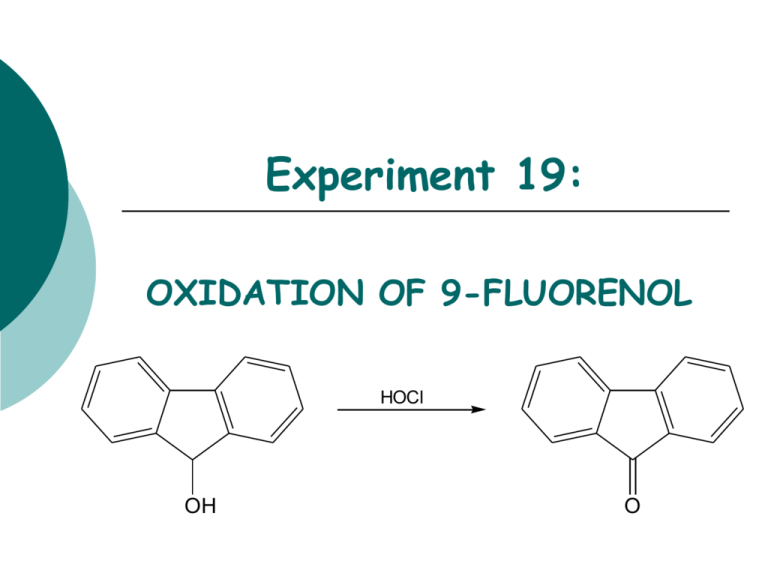
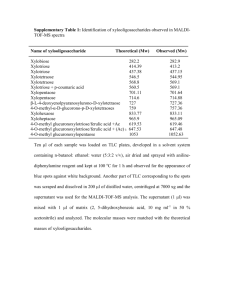
Experiment 19: OXIDATION OF 9-FLUORENOL HOCl OH O Objectives: To synthesize a ketone from a secondary alcohol using household bleach. To monitor the reaction progress using TLC analysis. To purify product using simple extraction. To analyze the purity of the product using TLC and melting point analysis. To characterize reactants and products using IR analysis. Before coming to lab… Review these techniques: TLC analysis Acid-base Extraction Drying over MgSO4 Melting Point Analysis TYPICAL OXIDATION VS. GREEN OXIDATION Typical oxidation using chromium compound Na 2Cr 2O7 OH H2O, CH 3CO 2H, heat O Greener oxidation using household bleach OH NaOCl, CH3CO2H acetone O THE OXIDIZING AGENT NaOCl + CH3CO2H HOCl + CH3CO2Na Sodium Hypochlorite • Sodium acetic acid hypochlorous acid sodium acetate hypochlorite is the main ingredient in bleach. • It must first be converted to hypochlorous acid in order to oxidize the alcohol. • HOCl is a source of a positive Cl, which has 2 fewer electrons than a chloride anion. • Remember, oxidation is the loss of H or the addition of O. PROPOSED MECHANISM HOCl H3O+ H Cl O - H2O + H H OH H O H Cl O - H3O+ Although complex, the mechanism results in the exchange of a Cl+ with a H+ on oxygen, followed by subsequent elimination of HCl to form the ketone. H H O O H H Cl H - H3O+ + H3O+ + Cl- O A FEW THINGS TO CONSIDER… HOCl OH O The 9-fluorenol can DONATE hydrogen bonds to the silica gel on the TLC plate, resulting in a lower TLC Rf value! The 9-fluorenone can ACCEPT hydrogen bonds, but not donate to the silica gel on the TLC plate, resulting in a higher TLC Rf value! x 1 x 2 x 3 A FEW THINGS TO CONSIDER… HOCl OH Less conjugated More conjugated O • The more conjugated a compound is, the higher the wavelength of light it absorbs. • The visible region of the spectrum is the 400-700 nm wavelength range. • If a compound absorbs light close to 400 nm, it will appear as a yellow color. EXPERIMENTAL PROCEDURE (SYNTHESIS) Add Fluorenol to 50 mL flask with stir bar. Add acetone and stir of until dissolved. Add acetic acid and bleach while stirring. Place a small cork in top of flask to reduce decomposition of bleach. Stir 10 minutes. Perform TLC experiment to check for completion. If oxidation is incomplete, add more acetic acid and bleach, react for 10 additional minutes and repeat TLC experiment. If oxidation is complete, proceed to purification steps. x 1 x 2 x 3 EXPERIMENTAL PROCEDURE (PURIFICATION) Transfer liquid to separatory funnel. Extract product into hexane. Wash organic layer with 5% NaHCO3 and Saturated NaCl. Transfer organic layer to flask. Dry over MgSO4. Transfer liquid to preweighed beaker. Evaporate solvent on hotplate. Place solid in warm oven for 10 min. Obtain final product mass, calculate % yield, and perform melting point analysis. Table 19.1 Calculate based on 9-fluorenol ONLY! Bleach is used in excess! Theoretical yield (g) Actual yield (g) % yield Melting Range (oC) Product Appearance compare to lit value of 81-85oC. Record the physical state and color of product. Table 19.2 Should be calculated based on 9-Fluorenol and hypochlorous acid (HOCl). Review Experiment 13 for calculation. Atom Economy Cost per gram Remember to calculate COST PER SYNTHESIS 1st based on all reagents and solvents! Table 19.3 TLC Rf values Compound Standards 9-fluorenone Rf values are UNITLESS! Rf values are 2 decimal places ONLY! If more than one TLC experiment is performed, record data from final TLC plate! 9-fluorenol Sample IR Spectroscopy OH O 9-fluorenol to concentrate on the types of bonds that indicate the CONVERSION from reactant to product! 9-fluorenone • Remember 3196 1031 1716 Table 19.4 Functional Group OH stretch C-O stretch C=O stretch Base Values (cm-1) 9-fluorenol 9-fluorenone SAFETY CONCERNS All compounds used in today’s experiment are FLAMMABLE and TOXIC! Use extreme caution when in use! • WASTE MANAGEMENT Place aqueous layer from extraction in container labeled “AQUEOUS WASTE (Ketones)”. Place TLC solvent in container labeled “ORGANIC WASTE (Ketones)”. Place used TLC and melting point capillaries in BROKEN GLASS CONTAINER. Place product in container labeled “9-FLUORENONE (Student Prep). CLEANING Clean all glassware with soap, water, and brush if necessary. Rinse all glassware with wash acetone before returning to lab drawer. DO NOT return any glassware to lab drawer dirty or wet.