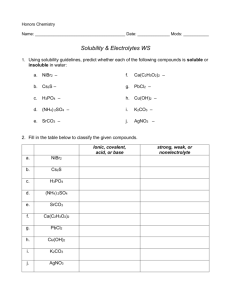
1) BellRinger: Name the following ionic compounds a) NaCl b) Li2S

1) BellRinger: Name the following ionic compounds: a) NaCl b) Li
2
S c) Mg
3
N
2
2) Worksheet #30
3) You must make up all work by this
Wednesday 1/13 for the semester.
Only exception is Test #6. Please see me if you will have a conflict with this.
4) Test #6 this Friday (1/15)
Binary Compounds
1) metal 2) left, right 3) first 4a) Cu b) Al c) Bi d) K e) Ta f) Li g) copper h) sodium i) barium j) aluminum k) lithium
5) IDE 6a) boride b) silicide c) oxide d) carbide e) nitride f) sulfide
7a) sodium sulfide b) lithium oxide c) magnesium bromide d) aluminum nitride
Binary Compounds
8a) Ca (+2) Cl (-1) Cl(-1) = CaCl
2 b) Ba (2+) S (2-) = BaS c) Cs (+1) Cs (+1) Cs (+1) N(3-) = Cs
3
N d) Li (+1) Br (-1) = LiBr
Compounds with Transition Metals
1) D, transition 2) valence 3) 2, 3
4) Fe (2+) Cl (-1) Cl (-1) = FeCl
2
SHOW CRISS CROSS METHOD:
Fe (2+) Cl (-1)
Fe Cl
2
Compounds with Transition Metals
5) Fe (3+) Cl (-1) Cl (-1) Cl (-1) = FeCl
3
SHOW CRISS CROSS METHOD
Fe (+3) Cl(-1)
Fe Cl
3
Polyatomic Ions
2) Na (+1) NO
3
(-1) = NaNO
3
3) Ca (+2) NO
3
(-1) NO
3
(-1) = Ca(NO
3
)
2
4a) NH
4
(+1) Cl (-1) = NH
4
Cl b) Mg (+2) OH (-1) OH(-1) = Mg(OH)
2 c) Be (+2) C
2
H
3
O
2
(-1) C
2
H
3
O
2
(-1) = Be(C
2
H
3
O
2
)
2 d) K (+1) OH (-1) = KOH e) Li (+1) C
2
H
3
O
2
(-1) = Li C
2
H
3
O
2 f) Na (+1) Na(+1) SO
4
(2-) = Na
2
SO
4
CLOSING ACTIVITY
1) SHOW WORK and give the formula for sodium carbonate
2) Name the following: LiOH