Lesson 3 – Description:
advertisement

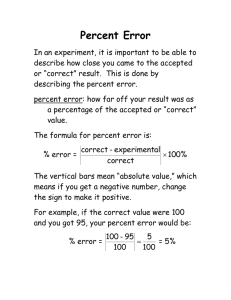
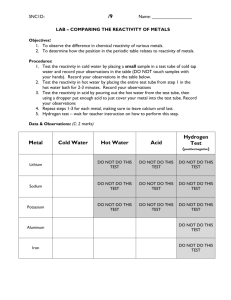
Lesson 3 – Description: The Chesapeake Bay’s Water: Students review how to determine properties of matter as physical, chemical, organic or non-organic. Student then look at the properties and uses of water. Unit Goals: Describe characteristics of the Bay water. Lesson Objectives: Describe some of the properties of water. Differentiate chemical and physical properties. Differentiate organic and non-organic compounds. How do we use water? Make a list of 5 different ways we use water. Some examples: Drinking, cooking, cooling, industrial chemicals, cleaning, irrigation, chemical reactions, recreation, … Why do we use water? So what makes water so special? Like most objects, the make up of water gives it is properties. The ability to sustain life, be used for industries, and even control seasonal changes. How do we determine the properties of water? Vocabulary Add terms that are bolded and underlined to your Vocabulary chart for the Chesapeake Bay. Describing & Classifying Matter Physical vs. Chemical Properties Describing Matter You must observe matter carefully to describe it well Characteristics that help you describe and identify matter are called properties. Properties may be physical or chemical. Describing & Classifying Matter Physical vs. Chemical Properties Physical Property: A property that can be observed or measured without changing the composition of matter. Physical properties are used to observe and describe matter. Describing & Classifying Matter Physical vs. Chemical Properties Physical properties can be subcategorized as qualitative or quantitative properties.\ Qualitative Property: Can be described with words and can’t be measured (Hint: Quality) Quantitative Property: Can be measured and expressed numerically (Hint: Quantity – how much?) Describing & Classifying Matter Physical vs. Chemical Properties Chemical Property: A property that can be observed when one kind of matter is converted into a different kind of matter. Example chemical property of iron: Reacts with oxygen to form rust Rust and iron have different physical and chemical properties! Physical Properties Qualitative Chemical Properties Quantitative Physical State Melting Point Water Reactivity Color Boiling Point Air Reactivity Odor Density Crystal Shape Solubility Malleability Electrical Conductivity Pure Substance Reactivity Ductility Thermal Conductivity Combustibility Oxygen Reactivity pH Reactivity Hardness Toxicity brittleness Decomposition Chemical Formula Describing & Classifying Matter Physical vs. Chemical Properties Chemical Formula A chemical formula is a way of writing out a chemical based on the elements/compounds it is made of. Example: Water = H2O = 2 Hydrogen + 1 Oxygen There are two basic types of compounds Organic Inorganic Organic vs. Inorganic Chemicals Organic compound are chemicals that: Are produced in biotic processes Contain the element carbon. Inorganic compounds are chemicals that: Do not contain the element carbon Do have carbon but do not have carbon-hydrogen bonds Are produced by abiotic processes. Organic and Inorganic Carbon Describing & Classifying Matter Physical vs. Chemical Properties Look at the terms on the “Physical or Chemical Properties” handout. With the chart on the next slide and using your vocabulary, try to determine if the characteristics are physical or chemical properties and if the chemicals are organic or inorganic. Physical Properties Qualitative Chemical Properties Quantitative Physical State Melting Point Water Reactivity Color Boiling Point Air Reactivity Odor Density Crystal Shape Solubility Malleability Electrical Conductivity Pure Substance Reactivity Ductility Thermal Conductivity Combustibility Oxygen Reactivity pH Reactivity Hardness Toxicity brittleness Decomposition Chemical Formula Why do we use water? Like most objects, the make up of water gives it is properties. The ability to sustain life, be used for industries, and even control seasonal changes. Follow along, answering your “HowStuffWorks: Water Video questions, as we observe how water functions. Feel free to ask questions during the video by raising your hand.