Chapter 8, Part 5
advertisement

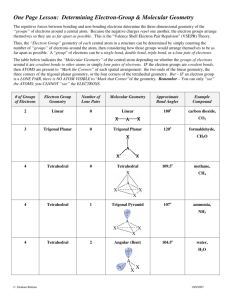
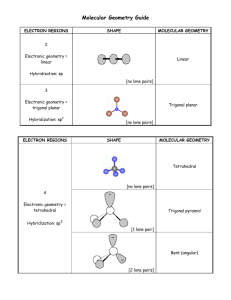
November 20, 2009 •C h a p t e r 8 •Second set of Chapt. 8 homework dates extended until after break •Lab Notes •Today’s Topic: Electron Distribution in Molecules (8.5) Molecular Shape (8.6) Molecular Polarity (8.7) Formal Charge and Oxidation Number Examples Partial Charges Charges Within Molecules Formal charge Electrons are shared evenly throughout molecule Closer to “real” charge than oxidation number Use to determine which nonequivalent resonance structure is more likely Oxidation Number Bonding electrons “belong” to more electronegative atom Quick and easy to determine without Lewis structure Doesn’t really reflect actual charges on atoms Use to determine whether compounds are oxidized/reduced in chemical reactions Partial Charges How electrons are really distributed in a molecule Need computer to calculate Used only when you need very accurate description- molecular modeling What is the formal charge on C in CN-? 1. -2 63% 2. -1 3. 0 4. +1 5. +2 12% 8% 1 2 3 6% 4 10% 5 What is the formal charge on C in CN-? 1. -2 20% 20% 20% 20% 20% 2. -1 3. 0 4. +1 5. +2 1 2 3 4 5 Molecular Structure Chemical Formula Lewis Structure #Structural Pairs (Valence ePairs) Electron Pair Geometry Molecular Geometry Lewis Structure # Structural Pairs # Structural Pairs = # bonded atoms Electron Geometry + # lone pairs Molecular Geometry on central atom # Structural Pairs determines Electron Pair Geometry # Structural Pairs determines Electron Pair Geometry # Structural Pairs determines Electron Pair Geometry What is the electron pair geometry of ozone? 1. 2. 3. 4. 5. Linear Trigonal planar Tetrahedral Trigonal bipyramidal octahedral 57% 21% 13% 9% 0% 1 2 3 4 5 What is the electron pair geometry of SCN-? 1. 2. 3. 4. 5. Linear Trigonal planar Tetrahedral Trigonal bipyramidal octahedral 77% 13% 9% 2% 1 2 3 4 0% 5 Molecular Geometry = Electron Pair Geometry, ignoring lone pairs BF3 and O3 Molecular Geometry = Electron Pair Geometry, ignoring lone pairs Molecular Geometry = Electron Pair Geometry, ignoring lone pairs Lone pairs always go in equatorial sites Molecular Geometry = Electron Pair Geometry, ignoring lone pairs What is the molecular geometry of NO2-? 1. 2. 3. 4. 5. 6. 7. 8. 9. Linear Bent Trigonal planar Tetrahedral Trigonal pyramid T-shaped Square pyramid Square planar octahedral 11% 11% 1 2 11% 11% 11% 11% 11% 3 4 5 6 7 11% 11% 8 9 What is the molecular geometry of … 1. 2. 3. 4. 5. 6. 7. 8. 9. Linear Bent Trigonal planar Tetrahedral Trigonal pyramid T-shaped Square pyramid Square planar octahedral 11% 11% 1 2 11% 11% 11% 11% 11% 3 4 5 6 7 11% 11% 8 9 What is the molecular geometry of … 1. 2. 3. 4. 5. 6. 7. 8. 9. Linear Bent Trigonal planar Tetrahedral Trigonal pyramid T-shaped Square pyramid Square planar octahedral 11% 11% 1 2 11% 11% 11% 11% 11% 3 4 5 6 7 11% 11% 8 9