Electron-Pair Geometry
advertisement
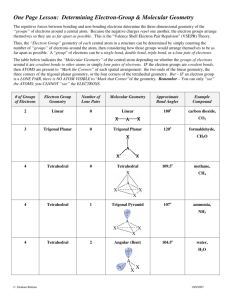
Mark S. Cracolice Edward I. Peters www.cengage.com/chemistry/cracolice Chapter 13 Structure and Shape Mark S. Cracolice • The University of Montana Drawing Lewis Diagrams 1 Count the total number of valence electrons. If the species is an ion, the number of valence electrons must be adjusted for the charge on the ion. Determine the number of electron pairs. 2 Place the least electronegative atom in the center of the molecule. 3 Draw a tentative diagram. Place one electron pair between each pair of bonded atoms. Drawing Lewis Diagrams 4 Determine the number of lone pairs by subtracting the number of single bonds from the total number of electron pairs available. 5 Starting at the outer atoms, distribute the available lone pair electrons to complete the octet around each atom except hydrogen which requires a duet. 6 If there are not enough electrons to complete all octets, move one or more lone pairs from an outer atom to form a double or triple bond with the central atom until all atoms have an octet. Drawing Lewis Diagrams Example: Draw the Lewis diagram for carbon tetrafluoride. First, count valence electrons. CF4: 4 (C) + 4 × 7 (F) = 32 valence electrons There are 16 electron pairs Second, determine the central atom by comparing electronagativity EN of C < EN of F, so C is central Drawing Lewis Diagrams Draw a tentative diagram with all single bonds and lone pairs.. Drawing Lewis Diagrams Check Carbon has an octet (4 bonding pairs = 8 electrons) Each fluorine has an octet (one bonding pair and three lone pairs) Valence Shell Electron-Pair Repulsion Theory, VSEPR. Electron pairs repel one another. Therefore they distribute themselves in positions around a central atom that are as far away from each other as possible. These are the locations of lowest potential energy. Electron-Pair Geometry 1. Count the number of lone electron pairs and the number of bonding electron pairs around the central atom. The total number of electron pairs is called the steric number, SN. 2. Distribute the electrons pairs in positions that are far away from each other as possible. These are the locations of lowest energy. Electron-Pair Geometry Electron-Pair Geometry Arrangement of regions of electron density (electron pairs) around a central atom in a molecule. Electron-Pair Angle The geometric angle formed by any two electron pairs and the central atom. Electron-Pair Geometry a. The central atom has two electron pairs the electron pair geometry is linear. The electron pair angle is 1800 b. The central atoms is surrounded by three electron pairs. The geometry is trigonal planar. The electron pair angle is 1200 c. The central atom is surrounded by four electron pairs, The geometry is tetrahedral, and the electron pair angle is 109.50 Molecular Geometry Molecular Geometry Arrangement of atoms around a central atom in a molecule. Molecular geometry follows from electron-pair geometry. Molecular Geometry: BeF2 Regions of electron density: 2 Electron-pair geometry: Linear Regions bonded: 2 Molecular geometry: Linear Molecular Geometry: BF3 Regions of electron density: 3 Electron-pair geometry: Trigonal planar Regions bonded: 3 Molecular geometry: Trigonal planar Molecular Geometry: SO2 Regions of electron density: 3 Electron-pair geometry: Trigonal planar Regions bonded: 2 Molecular geometry: Angular Molecular Geometry: CH4 Regions of electron density: 4 Electron-pair geometry: Tetrahedral Regions bonded: 4 Molecular geometry: Tetrahedral Molecular Geometry: NH3 Regions of electron density: 4 Electron-pair geometry: Tetrahedral Regions bonded: 3 Molecular geometry: Trigonal pyramidal Molecular Geometry: H2O Regions of electron density: 4 Electron-pair geometry: Tetrahedral Regions bonded: 2 Molecular geometry: Bent Molecular Geometry Molecular Geometry Conventions Used for Drawing Wedge-and-Dash Diagrams Polarity of Molecules Polar Molecule A molecule in which there is an asymmetrical distribution of charge. Nonpolar molecule A molecule in which there is a symmetrical distribution of charge. Polarity of Molecules In an electric field, polar molecules are oriented with the positive end toward the direction of the field. Polarity of Molecules Polarity of Molecules Homework • Prepare Exercise B