In-Class Questions
advertisement

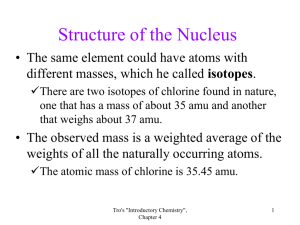
4.1 What is the charge on the electron, the proton, and the neutron, respectively? 1. 2. 3. 4. 5. Tro IC.3 -1, +1, +1 -1, +1, -1 -1, 0, +1 -1, +1, 0 +1, +1, 0 4.1 What is the charge on the electron, the proton, and the neutron, respectively? 1. 2. 3. 4. 5. Tro IC.3 -1, +1, +1 -1, +1, -1 -1, 0, +1 -1, +1, 0 +1, +1, 0 4.2 Which scientist discovered the electron? 1. 2. 3. 4. 5. Tro IC.3 Democritus J. J. Thomson John Dalton Lord Kelvin Ernest Rutherford 4.2 Which scientist discovered the electron? 1. 2. 3. 4. 5. Tro IC.3 Democritus J. J. Thomson John Dalton Lord Kelvin Ernest Rutherford 4.3 Which of the following subatomic particles has the least mass? 1. 2. 3. 4. 5. Tro IC.3 Electron Neutron Proton Two of the above All of the above 4.3 Which of the following subatomic particles has the least mass? 1. 2. 3. 4. 5. Tro IC.3 Electron Neutron Proton Two of the above All of the above 4.4 What subatomic particle(s) is/are found in the nucleus? 1. 2. 3. 4. 5. Tro IC.3 Electron Neutron Proton Two of the above All of the above 4.4 What subatomic particle(s) is/are found in the nucleus? 1. 2. 3. 4. 5. Tro IC.3 Electron Neutron Proton Two of the above All of the above 4.5 Which element has the chemical symbol Pb? 1. 2. 3. 4. 5. Tro IC.3 Mercury Polonium Platinum Tungsten Lead 4.5 Which element has the chemical symbol Pb? 1. 2. 3. 4. 5. Tro IC.3 Mercury Polonium Platinum Tungsten Lead 4.6 What is the chemical symbol for potassium? 1. 2. 3. 4. 5. Tro IC.3 Po P Pt Pb K 4.6 What is the chemical symbol for potassium? 1. 2. 3. 4. 5. Tro IC.3 Po P Pt Pb K 4.7 How many protons are in one silver atom? 1. 2. 3. 4. 5. Tro IC.3 47 60 94 108 154 4.7 How many protons are in one silver atom? 1. 2. 3. 4. 5. Tro IC.3 47 60 94 108 154 4.8 Which of the following elements is a metalloid? 1. 2. 3. 4. 5. Tro IC.3 Aluminum Silicon Tin Phosphorus Iodine 4.8 Which of the following elements is a metalloid? 1. 2. 3. 4. 5. Tro IC.3 Aluminum Silicon Tin Phosphorus Iodine 4.9 Which of the following elements is a metalloid? 1. 2. 3. 4. 5. Tro IC.3 Arsenic Bismuth Carbon Iodine Gallium 4.9 Which of the following elements is a metalloid? 1. 2. 3. 4. 5. Tro IC.3 Arsenic Bismuth Carbon Iodine Gallium 4.10 Strontium is in the family of: 1. 2. 3. 4. 5. Tro IC.3 Halogens Noble gases Alkaline earth metals Alkali metals Pnictogens 4.10 Strontium is in the family of: 1. 2. 3. 4. 5. Tro IC.3 Halogens Noble gases Alkaline earth metals Alkali metals Pnictogens 4.11 Chlorine is in the family of: 1. 2. 3. 4. 5. Tro IC.3 Halogens Noble gases Alkaline earth metals Alkali metals Pnictogens 4.11 Chlorine is in the family of: 1. 2. 3. 4. 5. Tro IC.3 Halogens Noble gases Alkaline earth metals Alkali metals Pnictogens 4.12 The total number of neutrons and protons in a given nucleus is referred to as the: 1. 2. 3. 4. 5. Tro IC.3 Proton number Mass number Atomic number Isotope number Neutron number 4.12 The total number of neutrons and protons in a given nucleus is referred to as the: 1. 2. 3. 4. 5. Tro IC.3 Proton number Mass number Atomic number Isotope number Neutron number 4.13 How many electrons are in the bromide ion, 81Br1–? 1. 2. 3. 4. 5. Tro IC.3 35 36 46 70 80 4.13 How many electrons are in the bromide ion, 81Br1–? 1. 2. 3. 4. 5. Tro IC.3 35 36 46 70 80 4.14 How many electrons, protons, and neutrons respectively are in the iron(III) ion, 56Fe3+ ? 1. 2. 3. 4. 5. Tro IC.3 23, 26, 26, 26, 29, 26, 26, 26, 26, 26, 30 30 56 26 30 4.14 How many electrons, protons, and neutrons respectively are in the iron(III) ion, 56Fe3+ ? 1. 2. 3. 4. 5. Tro IC.3 23, 26, 26, 26, 29, 26, 26, 26, 26, 26, 30 30 56 26 30 4.15 What is the symbol for an ion having 12 protons and 10 electrons? 1. 2. 3. 4. 5. Tro IC.3 Si2+ Si Mg2+ Ne Ne2– 4.15 What is the symbol for an ion having 12 protons and 10 electrons? 1. 2. 3. 4. 5. Tro IC.3 Si2+ Si Mg2+ Ne Ne2– 4.16 What is the symbol for an ion having 15 protons and 18 electrons? 1. 2. 3. 4. 5. Tro IC.3 Ph3– Ar P3+ Cl1– P3– 4.16 What is the symbol for an ion having 15 protons and 18 electrons? 1. 2. 3. 4. 5. Tro IC.3 Ph3– Ar P3+ Cl1– P3– 4.17 How many electrons are in one scandium ion, 45Sc3+? 1. 2. 3. 4. 5. Tro IC.3 45 42 48 24 18 4.17 How many electrons are in one scandium ion, 45Sc3+? 1. 2. 3. 4. 5. Tro IC.3 45 42 48 24 18 4.18 Which atom has the same number of protons as 59Ni? 1. 2. 3. 4. 5. Tro IC.3 59Fe 58Ni 59Co 141Pr 102Rh 4.18 Which atom has the same number of protons as 59Ni? 1. 2. 3. 4. 5. Tro IC.3 59Fe 58Ni 59Co 141Pr 102Rh 4.19 The nucleus of a fluorine-19 atom contains: 1. 2. 3. 4. 5. Tro IC.3 19 protons and 19 electrons 19 protons and 0 electrons 9 protons and 10 electrons 19 protons and 19 neutrons 9 protons and 10 neutrons 4.19 The nucleus of a fluorine-19 atom contains: 1. 2. 3. 4. 5. Tro IC.3 19 protons and 19 electrons 19 protons and 0 electrons 9 protons and 10 electrons 19 protons and 19 neutrons 9 protons and 10 neutrons 4.20 A fluorine-19 atom is symbolized as: 1. 2. 3. 4. 5. Tro IC.3 9F 10 9F 10 10F 9 19F 9 19F 10 4.20 A fluorine-19 atom is symbolized as: 1. 2. 3. 4. 5. Tro IC.3 9F 10 9F 10 10F 9 19F 9 19F 10 4.21 What is the charge of a halide ion? 1. 0 2. 1– 3. 1+ 4. 2– 5. 2+ Tro IC.3 4.21 What is the charge of a halide ion? 1. 0 2. 1– 3. 1+ 4. 2– 5. 2+ Tro IC.3 4.22 Which of the following species has 10 electrons and 14 neutrons? 1. 2. 3. 4. 5. Tro IC.3 27Al3+ 27Al 14N3– 24Mg2+ 20Ne 4.22 Which of the following species has 10 electrons and 14 neutrons? 1. 2. 3. 4. 5. Tro IC.3 27Al3+ 27Al 14N3– 24Mg2+ 20Ne 4.23 What is the charge of an oxygen atom? 1. 2. 3. 4. 5. Tro IC.3 0 1– 1+ 2– 2+ 4.23 What is the charge of an oxygen atom? 1. 2. 3. 4. 5. Tro IC.3 0 1– 1+ 2– 2+ 4.24 Isotopes have: 1. The same number of protons, but different numbers of neutrons 2. The same number of protons and neutrons 3. The same chemical symbol 4. The same number of electrons and neutrons 5. Two of the above Tro IC.3 4.24 Isotopes have: 1. The same number of protons, but different numbers of neutrons 2. The same number of protons and neutrons 3. The same chemical symbol 4. The same number of electrons and neutrons 5. Two of the above Tro IC.3 4.25 Which of the following elements has the most similar reaction with water as potassium? 1. 2. 3. 4. 5. Tro IC.3 Ca Ar Fe Na C 4.25 Which of the following elements has the most similar reaction with water as potassium? 1. 2. 3. 4. 5. Tro IC.3 Ca Ar Fe Na C 4.26 Carbon has three naturally occurring isotopes: C-12 with mass 12.000 amu and a natural abundance of 98.89%, C-13 with mass 13.004 amu and a natural abundance of 1.110%, and C-14 which is negligibly small. Calculate the atomic mass of carbon. 1. 2. 3. 4. 5. Tro IC.3 12.00 amu 12.01 amu 13.00 amu 14.00 amu 25.00 amu 4.26 Carbon has three naturally occurring isotopes: C-12 with mass 12.000 amu and a natural abundance of 98.89%, C-13 with mass 13.004 amu and a natural abundance of 1.110%, and C-14 which is negligibly small. Calculate the atomic mass of carbon. 1. 2. 3. 4. 5. Tro IC.3 12.00 amu 12.01 amu 13.00 amu 14.00 amu 25.00 amu 4.27 A hypothetical element, Hy, has three isotopes, Hy299, Hy-300, and Hy-301, which have the following natural abundance: 5.00 % (Hy-299), 65.00 % (Hy300), and 30.00 % (Hy-301). The atomic masses or the isotopes are 299.0 amu, 300.0 amu, and 301.0 amu, respectively. Calculate the atomic mass of Hy. 1. 2. 3. 4. 5. Tro IC.3 299.5 amu 299.8 amu 300.3 amu 300.6 amu 301.1 amu 4.27 A hypothetical element, Hy, has three isotopes, Hy299, Hy-300, and Hy-301, which have the following natural abundance: 5.00 % (Hy-299), 65.00 % (Hy300), and 30.00 % (Hy-301). The atomic masses or the isotopes are 299.0 amu, 300.0 amu, and 301.0 amu, respectively. Calculate the atomic mass of Hy. 1. 2. 3. 4. 5. Tro IC.3 299.5 amu 299.8 amu 300.3 amu 300.6 amu 301.1 amu 4.28 Which of the following is characteristic of metals? 1. 2. 3. 4. 5. Tro IC.3 Solid at room temperature Malleable Ductile Located toward the left side of the periodic table All of the above 4.28 Which of the following is characteristic of metals? 1. 2. 3. 4. 5. Tro IC.3 Solid at room temperature Malleable Ductile Located toward the left side of the periodic table All of the above 4.29 1. 2. 3. 4. 5. Tro IC.3 Which scientist discovered the nucleus? Robert Millikan J. J. Thomson John Dalton Lord Kelvin Ernest Rutherford 4.29 1. 2. 3. 4. 5. Tro IC.3 Which scientist discovered the nucleus? Robert Millikan J. J. Thomson John Dalton Lord Kelvin Ernest Rutherford