Cation Exchange Capacity (CEC)
advertisement

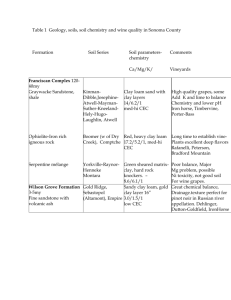
MANAJEMEN KESUBURAN TANAH KAPASITAS TUKAR KATION & HARA TANAMAN Cation Exchange Capacity (CEC) Clay Particles and Humus - affect chemical properties of soil - complex structures with many negative charge sites - negative charge sites attract positive ions called cations KTK = CEC Negative charge sites are referred to as . . . Cation exchange sites + attract cations from soil solution+ KTK = CEC Force of attraction is called: Adsorption similar to force of a magnet holding iron filings ADSORPSI = JERAPAN KATION KTK = CEC Cations can move on and off particles . . . when one leaves, another replaces it This process is called cation exchange, and cations involved are said to be exchangeable http://www.une.edu.au/~agronomy/SSCATXCH. dcr KTK = CEC The number of sites that a colloid (small particle) of charged clay or humus (micelles) contains is measured by the: Cation Exchange Capacity expressed in mEq/100g (older unit) or cmolc/kg KTK = CEC may range from: 2.0 mEq/100g for sand to > 50 mEq/100g for some clays and humus 100-300 mEq/100g under certain soil conditions KTK = CEC How fertile can a soil be? Does applying more fertilizer always provide more nutrients to plants? How much of the CEC is actually filled with cations? KTK = CEC The proportion of the CEC occupied by basic (+) nutrients such as Ca, Mg, K, Na, is called: Percent Base Saturation and is an indication of the potential CEC of a given soil KTK = CEC Estimations that > 99% of cations in soil solution are adsorbed . . . does not mean that percent base saturation is 99% KTK = CEC Example: A soil with CEC of 10 mEq/100g has 6 mEq/100g of bases (Ca, Mg, K, Na) occupying exchange sites What is the percent base saturation of the soil? KTK = CEC 6 mEq/100g bases 10 mEq/100g sites = 60 % base saturation KTK = CEC Cation Exchange is determined by: 1) strength of adsorption 2) law of mass KTK = CEC Strength of adsorption is as follows: H+ and Al3+ > Ca2+ > Mg2+ > K+ > NH4+ > Na+ KTK = CEC Law of Mass the more of one ion available, the greater the chance of adsorption KTK = CEC Cation Exchange Capacity (CEC) is the ability of the soil to hold onto nutrients and prevent them from leaching beyond the roots. The more cation exchange capacity a soil has, the more likely the soil will have a higher fertility level. When combined with other measures of soil fertility, CEC is a good indicator of soil quality and productivity. The cation exchange capacity of a soil is simply a measure of the quantity of sites on soil surfaces that can retain positively charged ions by electrostatic forces. Cations retained electrostatically are easily exchangeable with other cations in the soil solution and are thus readily available for plant uptake. Thus, CEC is important for maintaining adequate quantities of plant available calcium (Ca++), magnesium (Mg++) and potassium (K+) in soils. Other cations include Al+++( when pH < 5.5) , Na+, and H+. DIUNDUH DARI: http://www.swac.umn.edu/classes/soil2125/doc/s12ch2.htm ….. 17/9/2012 Cation exchange capacity (CEC) The capacity of a soil to adsorb and exchange cations (positively charge ions, Ca2+, Mg2+, K+, Na+, NH4+ , Al[OH]2 +, Al3+, and H+). This capacity is due to the net negative charge of soil colloids (clays and organic matter) DIUNDUH DARI: http://www.swac.umn.edu/classes/soil2125/doc/s12ch2.htm ….. 17/9/2012 Cation Exchange Capacity (CEC) Sources of charge on clays: 1.Ionizeable H+ on edges (pH-dependent, similar to charge on OM), just as in the case of a weak acid. 2.Isomorphous substitution in clays: • Substitution of Al3+ for Si4+ in the tetrahedral layer of clays • Substitution of Mg2+ for Al3+ in the octahedral layer of clay • This type of CEC is often referred to as permanent charge CEC because it is not affected by pH. Cation Exchange Capacity (CEC) Sources of charge on clays: 1. Both ionizable H+ and isomorphous substitution impart CEC to clays. 2. Total CEC of the soil is dependent upon the amount of these sources and also upon the surface area of clays exposed (lower when they clamp shut) 3. See swarm of cations in diffuse double layer Silicate Clay Types Amorphous silicate clays (allophane): 1. Mixtures of Al and Si that have not crystallized. May contain other oxides like Fe. 2. Often present where weathering ins not complete, as in from volcanic ash (present in Andisols) 3. CEC: variable to high, can have AEC (amphoteric), high affinity for P 4. Shrink-swell: low Silicate Clay Types Kandites (kaolinite, nacrite, halloysite): 1. Kaolinite is most common 2. Secondary mineral formed in soil; prevalent in highlyweathered soils 3. Structure: 1:1 , 0.7 nm spacing 4. Interlayer: hydrogen bonds between sheets (no water or cations) 5. CEC: low 6. Shrink-swell potential: none Silicate Clay Types Smectities (montmorillonite, saponite): 1. Secondary mineral formed in soil; prevalent in less highly weathered soils 2. Structure: 2:1 , 1-2 nm spacing 3. CEC: very high (highest of all clays) 4. Interlayer: water molecules and miscellaneous cations 5. Shrink-swell potential: very high Silicate Clay Types Hydrous mica and illite: •Poorly defined group, 2:1 clays •Micas: 1. Primary mineral (Important in igneous and metamorphic rocks) 2. Structure: 2:1 , 1 nm spacing 3. Interlayer: K+ 4. CEC: low 5. Shrink-swell potential: none Silicate Clay Types Hydrous mica and illite: 1. Fine-grained micas (formerly called illite): 2. Weathered mica (smaller particle, less interlayer K+) 3. Structure: 2:1, 1 nm spacing 4. CEC: intermediate 5. Interlayer: K 6. Shrink-swell potential: none Silicate Clay Types Vermiculite: 1. Secondary mineral formed in soil; prevalent in less highly weathered soils 2. Like fine-grained mica but no interlayer K+ 3. Structure: 2:1, 1.0 to 1.5 nm spacing 4. CEC: high 5. Interlayer: water molecules and miscellaneous cations, especially Mg 6. Shrink-swell potential: high, but less than smectite Silicate Clay Types Chlorite 1. Secondary mineral formed in soil 2. Structure: 2:1, 1.4 nm spacing 3. CEC: intermediate 4. Interlayer: Mg hydroxide octahedral sheet, firmly bonded 5. Shrink-swell potential: very low Silicate Clay Types Sesquioxides 1. Mixtures of Al, Fe oxides and hydroxides left after extensive weathering (hot humid soils - Oxisols, Ultisols) 2. Shrink-well: none 3. CEC: low, amphoteric can have AEC, high affinity for P Silicate clays: permanent charge CEC Vermiculite (High CEC, Mica (Primary mineral) 1.0 nm expands/contracts somewhat) SiO 4 Al(OH) 3 ≈1.4 nm K K K K K K K Ca Mg H 2 O Ca H 2 O Smectite (or Montmorillonite Illite (Med. CEC) (High CEC, expands/contracts a lot) ≈1.8 to 4.0 1.0 nm H+ K K K H+ H+ nm Ca Mg H Chlorite (Low-Med CEC) 2 O Ca H Kaolinite 0.72 0.93 nm nm H+ bonding 2 O KATION TUKAR The replacement of one adsorbed cation for another from solution. A simple example: Ca2+ exchange displaces exchangeable Na+ - ..Na+ 2+ ..Ca - [Ca2+] ..Na+ Dissolved in soil solution Negatively-charged clay XNa 2 + + Ca2+ XCa X = exchangeable 2+ + 2Na+ [Na+] [Na+] Cation Exchange Capacity (CEC) 1. Quantity of exchangeable cations per unit weight of soil 2. Strongly affect soil solution (through cation exchange) and are available to plants 3. Units: centimoles of charge refers to charge; so for example 1 centimole of Ca2+ has 2 centimoles of charge, whereas one centimole of K+ has 1 centimole of charge. 1. 2. 3. 4. Cation Exchange Strength of cation adsorption (lyotropic series): Na+ < K+ = NH4+ < Mg2+ = Ca2+ < Aln+ < H+ Adsorption depends on charge density (charge/vol), so increases with valence and decreases with size. Not all exchangeable ions are Aln+ and H+ because mass action allows the others to be present; but at equal soil solutoin conc's, this will be the order. DIUNDUH DARI: http://www.swac.umn.edu/classes/soil2125/doc/s12ch2.htm ….. 17/9/2012 Note: Al3+ is a weak acid and combines with water to form various ions depending on pH: pH < 4.5 pH 4.5-6.5 (mostly monovalent form) pH 6.5-8 (gibbsite) pH 8-11 3+ 2+ + 0 Al(H2O)6 <->Al(H2O)5(OH) <-> Al(H2O)4(OH)2 <-> Al(H2O)3(OH)3 <-> Al(H2O)2(OH)4- DIUNDUH DARI: http://hubcap.clemson.edu/~blpprt/acid1.html ….. 17/9/2012 AKSI MASA 1. 2. 3. Displacement of one adsorbed/exchangeable cation by another by competition for sites when the second has a high number of ions in solution (high concentration) This is why fertilization with K, Mg and liming (Ca2+) work - they flood exchange sites and drive off other even more strongly adsorbed cations (like H+ and Al). Also, sodic soils (10-20% exchangeable Na) are cured by gypsum in the same way. Diunduh dari: http://www.fao.org/docrep/field/003/AC172E/AC172E05.htm .... 17/9/2012 Ca2+ Displaces Al3+ by Mass Action even though Al3+ is more strongly absorbed Ca2 Al3+ Al3+ Al3+ + 2+ 2+ Ca Ca Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ 2+ Ca Al3+ Ca2+Ca2+ 2+ Ca Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Silicate clays: permanent charge CEC Vermiculite (High CEC, Mica (Primary mineral) 1.0 nm expands/contracts somewhat) SiO 4 Al(OH) 3 ≈1.4 nm K K K K K K K Ca Mg H 2 O Ca H 2 O Smectite (or Montmorillonite Illite (Med. CEC) (High CEC, expands/contracts a lot) ≈1.8 to 4.0 1.0 nm H+ K K K H+ H+ nm Ca Mg H Chlorite (Low-Med CEC) 2 O Ca H Kaolinite 0.72 0.93 nm nm H+ bonding 2 O HUMUS TANAH 1. Temporary (will ultimately decompose) 2. Nearly insoluble in water, but soluble in base (high pH) 3. Contains 30% each of proteins, lignin, complex sugars 50% C and O, 5% N 4. Very high CEC on a weight basis 5. Develops a net negative charge due to the dissociation of H+ from fenolic (-OH), carboxyl (-COOH), and phenolic ( -OH) groups as pH increases (solution H+ concentration decreases): pH-dependent CEC on Organic Matter No charge CEC and exch. K+ (could be any cation) R-OH0 + OH- --------> R-O- …K+ + H2O (R stands for one of the above groups) This leaves a net negative charge on the organic colloid (R-O-) which attracts cations just as the net negative charge on an isomorphously-substituted clay does. Organic matter is the most important source of pH-dependent CEC in soils. Organic matter : pH-dependent CEC OH O - K + + OHOH Low pH, sites protonated no CEC + H2O OH High pH (depronotated, cation exchange site) Measurement of Cation Exchange Capacity (CEC) and Base Saturation (%BS) 1. CEC is measured by applying concentrated ammonium chloride (NH4Cl) or ammonium acetate (NH4OAc) to the sample to exchange all exchangeable cations with NH4+ by mass action 2. The extractant solution is analyzed for Ca2+, Mg2+, K+, Na+, and in some cases Al to determine what was on the exchanger. 3. At that point, one measure of CEC can be made (see 1 below). Then the NH4+ is displaced by another cation (typically Na+ or K+ ) by mass action, and NH4+ is then measured to obtain another estimate of CEC. Measurement of CEC and %BS 1. The usual assumption is that NH4+ constitutes a negligible proportion of CEC. 2. Exchangeable NH4+ is often measured separately using concentrated KCl extractant. 3. H+ (pH) is not measured on this extractant, either; exchangeable H+ is measured another way. 4. Some soil scientists argue that there is no exchangeable H+ on mineral soils; all H+ that becomes absorbed onto clay minerals quickly enters the lattice structure and causes clay decomposition to hydrous oxides. There are three ways to measure CEC (two from one method and one from another method): 1. Sum of cations Method: • • • The sum of Ca2+, Mg2+, K+, Na+, and Al after extraction with 1M NH4Cl (a neutral salt which does not buffer pH). CEC by sum of cations, CECsum, and is measured in the first extractant in Figure 1. In a pure clay system (no organic matter Fe, Al hydrous oxides, of allophane; i.e., no pH-dependent CEC) this represents CEC and cations on the clay minerals (permanent charge CEC). Step 2. Displace exchangeable NH4+ + + with Na or K Step 1. Displace exchangeable cations with NH4+ 1 M NaCl of KCl 1 M NH 4Cl Soil Sample Soil Sample + Na +or K displaces exchangeable NH +4 + NH 4displaces exchangeable cations Extractant Extractant 2+ Analyze for Ca, K, Mg, 3+ gives Na, + and Al ; this exchangeable cations. Sum of these cations = CEC sum - -- Ca -- Mg -- K -- Na -- Al -- H 2+ 2+ + + 3+ + + NH + 4 + 2+ + Analyze for NH ; this4 gives CEC eff - -- NH -- NH -- NH -- NH -- NH -- NH + 4 + +4 4+ 4 + 4+ 4 Extractant (Exchangeable Cations, CEC sum) Figure 1. Measurement of exchangeable cations and CEC using neutral salt. (KCl) + Na + - -- Na -- Na -- Na -- Na -- Na -- Na Extractant (CEC eff) + + + + + + 2. Effective CEC (CECeff) at existing soil pH. 1. This includes the permanent charge CEC plus that portion of pH-dependent CEC that is in effect at existing soil pH. 2. It is determined from the second extractant in Figure 1, After the 1M NH4Cl extraction, the soil is washed with ethanol to remove soluble NH4+ , and then extracted with 1M NaCl to displace the exchangeable NH4+. 3. The extractant is analyzed for NH4+ . Step 2. Displace exchangeable NH4+ + + with Na or K Step 1. Displace exchangeable cations with NH4+ 1 M NaCl of KCl 1 M NH 4Cl Soil Sample Soil Sample + Na +or K displaces exchangeable NH +4 NH 4+displaces exchangeable cations Extractant Extractant 2+ Analyze for Ca, K, Mg, Na,+ and Al ; 3+ this gives exchangeable cations. Sum of these cations = CEC sum - -- Ca -- Mg -- K -- Na -- Al -- H 2+ 2+ + + + 3+ + + NH 4 + 2+ + Analyze for NH ; this 4 gives CEC eff - -- NH -- NH -- NH -- NH -- NH -- NH + 4 + +4 4+ 4 + 4+ 4 + Na Extractant (Exchangeable Cations, CEC sum) Figure 1. Measurement of exchangeable cations and CEC using neutral salt. (KCl) + - -- Na + -- Na + -- Na + -- Na + -- Na + -- Na + Extractant (CEC eff ) 3. Ammonium acetate CEC (CECOAc). 1. This includes permanent charge CEC + all pHdependent CEC. Is is measured by extracting the soil with either ammonium acetate (NH4OAc, buffers pH at 7.0). (Figure 2). 2. Then the same produre is followed as for the neutral salt CEC. 3. Note: exchangeable Al should be measured separately because Al precipitates as Al(OH)3 at high pH Step 2. Displace exchangeable NH4+ with Na + Step 1. Displace exchangeable cations with NH4+ 1 M NaCl 1 M NH4 OAc Buffers pH at 7 Soil Sample Soil Sample Na + displaces exchangeable + NH 4 NH 4+displaces exchangeable cations Extractant Extractant 2+ 2+ + Analyze for Ca, K, Mg, + and Na ; this gives exchangeable cations except for Al. 3+ - -- Ca 2+ -- Mg 2+ -- K + -- Na + -- Al 3+ -- H + + + NH 4 + Analyze for NH ; this 4 gives CEC pH 7 - -- NH -- NH -- NH -- NH -- NH -- NH Extractant (Exchangeable Cations) + 4 + +4 4 + 4 + 4+ 4 + Na + - -- Na + -- Na + -- Na + -- Na + -- Na + -- Na + Extractant (CEC) Figure 2. Measurement of exchangeable cations and CEC buffering pH at 7 using ammonium acetate. Figure 3. Types of CEC depend on how it is measured CECOAc CECeff CECsum Permanent Charge CEC pH-dependent CEC CECsum: Measured as the sum of Ca + Mg + K + Na + Al extracted with ammonium chloride in the first extraction in Figure 1 CECeff: Measured with ammonium chloride, neutral salt, after second extraction in Fig 1 CECOAc: Measured with ammonium acetate at pH 7 in Figure 2 Base Saturation. Base Cation Saturation Percentage (BCSP) (often stated as simply base saturation) BCSPis defined as the sum of exchangeable base cations (Ca2+, Mg2+, K+, and Na+) divided by CEC. It is usually expressed as a percentage of CEC thus: BCSP (%) (or %BS) = Ca + Mg + K + Na x100 CEC KEJENUHAN BASA 1. Since CEC can be measured in different ways, BCSP will vary with the method used, and must be specified. 2. For a soil with a given amount of exchangeable bases, % Base saturation calculated from CECsum will be greater than that calculated from CECeff which will be greater than that calculated from CECtot because more of the potential acidity on the pH-dependen CEC is counted as CEC (i.e., CECsum < CECeff < CECtot). 3. The example in Figure 4 shows how this might occur. In each case, the base cations are the same (6 cmolc kg-1); only the measure of CEC (the deminator) changes. Figure 4. BCSP value depends on which CEC measure is used CECOAc= 10 cmolc kg-1 CECeff = 8 cmolc kg-1 CECsum = 7 cmolc kg-1 Ca2+ Base Cations + Mg2+ + K+ + Na+ = 6 cmolc kg-1 Ca2+ + Mg2+ + K+ + Na + BSCPsum=________________________ CECsum = X 100 2+ + Mg2+ + K+ + Na + Ca __________________________ Ca2+ + Mg2+ + K+ + Acid cations Aln+ = 1 cmolc kg-1 H+ = 3 cmolc kg-1 X 100 6 7 = Na++ Aln+ Ca2+ + Mg2+ + K+ + Na + BSCPsum=________________________ X 100 CECrff Ca2+ + Mg2+ + K+ + Na + BSCPOAc=________________________ CECOAc = X 100 = 6 8 X 100 = 85% X 100 = 75% 6 10 X 100 = 60% Anion adsorption and retention on soils: Negatively-charged ions adsorbed on positively-charges sites. •In general, anion adsorption is associated with allophane and the hydrous oxides of Fe and Al in soils. •H2PO4- >> SO4-2- >> NO3- > Cl- (the latter being nil in all but the most sequoixide-rich soils) •Anion adsorption on these surfaces is highly dependent upon pH. •Usually much lower than CEC in temperate, nonvolcanic ash soils. Allophane, Fe and Al hydrous oxides are amphoteric : they take on different charges depending upon pH. + OH 2 Al Cl - - OH Al OH Low pH (protonated, anion exchange site) O K+ Al OH Zero Point of Charge OH High pH (depronotated, cation exchange site) pH pH is the negative log of the H+ activity = -log (H+); therefore, 10-pH = (H+) (in moles L-1) Soil reaction, or pH is taken in a paste of water or 0.01 CaCl2. The latter gives a lower pH than the former, in most cases, because the Ca displaces exchangeable H and Al by mass action. pH pH decreases as base saturation decreases (recall that you must keep the methods constant, that is by sum, eff, or Oac; the soil in Figure 4 has only one pH although base saturation value differs by method). pH has a strong effect on plant growth (Fig 4-10) and nutrient availability (Fig 4-11) It not only changes the solubility of many nutrients (will be reviewed later), but may also cause direct toxicity (Al, usually) to plant roots. Buffering capacity: •Ability of the soil (or whatever else) to resist changes in pH. •In soils, this is a function of exchangeable H and Al in acid soils and carbonates in alkaline soils. •CEC always plays a major role in buffering. Potential Acidity Buffering Active Acidity •Total acidity on solid phase > 10,000 x that in soil solution HARA TANAMAN There are at least 17 elements recognized as essential nutrients for plants; we will recognize 18 elements: C, H, O, P, K, N, S, Ca, Fe, Mg, Mn, Mo, Cl, Cu, Zn, B, Co, Ni HARA TANAMAN Nutrients grouped into 2 categories according to the relative amount used by plants: Macronutrients – major elements; large amounts Micronutrients – minor elements; small amounts Both are essential for optimal plant production PENYERAPAN HARA OLEH BULU AKAR Diunduh dari: http://www.waldeneffect.org/blog/Cation_exchange_capacity/ ….. 17/9/2012 PERTUKARAN KATION HARA HARA TANAMAN Plants obtain some mineral nutrients through ion exchange between the soil solution and the surface of clay particles. Diunduh dari: http://bcs.whfreeman.com/thelifewire8e/content/cat_010/3601001.htm?v=chapter&i=36010.01&s=36000&n=00010&o=|01000| ….. 17/9/2012 HARA TANAMAN Cation exchange in soil. Clay soils are usually alkaline and bind positively charged minerals (cations such as Ca2+). Hydrogen ions (H+) help make nutrients available by displacing the cations. Plants secreting H+ by cellular respiration: CO2 reacts with H2O to form carbonic acid (H2CO3) in the soil, which dissociates to add H+ to the soil. Diunduh dari: http://bio1903.nicerweb.com/Locked/media/ch37/soil_availability.html ..... 17/9/2012 HARA TANAMAN Except for C, H, O . . . - Nitrogen (N) is present in greatest concentrations; - Plants respond readily to Nitrogen (N) KTK TANAH In most soils, 99% of soil cations can be found attached to micelles (clay particles & organic matter) and 1% can be found in solution. Cations in the soil (mainly Ca++, Mg++, K+ and Na+) maintain an equilibrium between adsorption to the negative sites and solution in the soil water. This equilibrium produces exchanges -- when one cation detaches from a site (leaving it free), another cation attaches to it. Therefore the negatively charged sites are called cation exchange sites. The total number of sites is the Cation Exchange Capacity or CEC Cation Exchange Capacity 1) the number of cation adsorption sites per unit weight of soil or 2) the sum total of exchangeable cations that a soil can adsorb. * CEC is expressed in milliequivalents (meq) per 100 g of oven dry soil. Equivalent weight = molecular or atomic wt (g) valence or charges per formula Milliequivalent (MEQ) 1 meq wt. of CEC has 6.02 x 10 20 adsorption sites MEQ of Common Cations Element Na+ K+ Ca++ Mg++ Valence 1 1 2 2 Eq. Wt 23/1=23 39/1=39 40/2=20 24/2 = 12 MEQ wt .023 .039 .02 .012 Sample calculation for equivalent weight for lime or CaCO3 CaCO3 - formula wt. = 40 + 12 + 48 = 100 charges involved = 2 eqwt. = 50 meq = .05 grams Or one meq of Lime = .05grams Calculation of CEC with % clay and % OM Assume Avg CEC for % OM = 200 meq/100g Assume Avg CEC for % clay = 50 meq/100g CEC = (% OM x 200) + (% Clay x 50) From soil data: soil with 2% OM and 10% Clay 200 x .02 + 50 x .1 = 4 + 5 = 9 meq/100 g Predicting CEC 1) sum of cations : remove all cations and total the amount 2) NH4+ saturation: soil is saturated with NH4+ - the NH4+ is replaced by Ca++ and the NH4+ removed is measured. 3) Estimation based on texture: Sand = 0-3 meq/100 g LS to SL = 3-10 Loam = 10 - 15 Clay Loam = 15-30 Clay = > 30 (depends on kind of clay) A high CEC value (>25) is a good indicator that a soil has a high clay and/organic matter content and can hold a lot of cations. Soil with a low CEC value (<5) is a good indication that a soil is sandy with little or no organic matter that cannot hold many cations. http://www.spectrumanalytic.com/support/library/ff/CEC_BpH_and_percent_sat.htm Base Saturation vs pH ÷ CEC x 100 - meq H ÷ CEC x100 % Base Saturation - meq bases % Hydrogen Saturation Example: Ap Soil Horizon Cations-- H+ Ca++ Mg++ K+ Na+ 9.4 14 3 0.5 CEC = 27 meq/100g (sum of cations) 0.1 ÷ 27 x 100 = 65% % hydrogen sat = 9.4÷27 x100 = 35% % base sat = 17.6 pH vs. Base Saturationan approximate relationship Buffering Capacity The ability of soil to resist change in pH. The amount of H+ in the soil solution is small compared with the “H+, Al + 3 ” adsorbed on the soil colloids (reserve) Neutralization (by the addition of bases) of the solution H+ (H+ is removed from the system) results in a rapid replacement of H+ from the exchangeable H+ on the soil colloid. CaCO3 when added to soil will neutralize H+. CaCO3 = Lime (dolomitic = MgCO3 & CaCO3 Sample % BS Problem Calculate the amount of CaCO3 which must be added to an acre furrow slice of this soil to raise the soil’s base saturation to 90% SOIL = CEC of 17meq/100g and BS = 32% (hint = takes 1000 lbs CaCO3/acre to neutralize 1 meq of H+/100 g 90% 32% = 58% change in BS 0.58 x 17 meq/100g = 9.86 meq/100g of H+ to neutralize or 9.86/100 X 1000 lbs CaCo3/100g = 9860 lbs OR 9.86 meq x .05g/meq = .493g/100g and .493/100g is to X / 2,000,000lbs or X = 9860 lbs. Divided by 2000 lb/ton = 4.9 tons SOIL = CEC of 27meq/100g and BS = 32% 90-32=58%change in base or .58x27=15.66 me of H+ to neutralize 15.66x1000lbs=15660/2000lbs/ton= 7.8tons In the Southeast US, if fertilizer and lime is applied to raise the base saturation of a kaolinitic soil to 85 percent as commonly done in the Midwest, the resulting pH would be between 7.1 and 7.5- due to low CEC from Kaolinite Soil pH values in that range would result in a major problem with zinc and manganese deficiency. Thus, soils are only limed to 60-70% BS. Tifton soils formed in loamy sediments of marine origin. Cotton, peanuts,soybeans, and corn are the principal crops grown on these soils in Georgia CEC and Soil Testing: Because the CEC of a soil is relatively constant unless large amounts of organic matter are added, it is not measured or reported with a routine soil test. Ca : Mg Ratio and Soil Testing Some soil testing labs will report ideal calcium to magnesium ratios for plant growth. However, most plants tolerate a very wide range of soil calcium to magnesium ratios. Adjusting the ratios of calcium and magnesium on the exchange complex by adding gypsum (calcium sulfate) or Epsom salts (magnesium sulfate) has not been shown to significantly benefit plant growth. Gypsum is primarily used as a soil amendment to improve water penetration and increase the level of calcium in the soil.