Study Guide Answer Key
advertisement

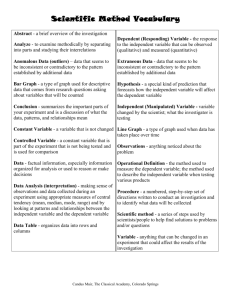
Thermal Energy Study Guide 1) What instrument and units are used to measure temperature? Thermometer, Celsius most commonly used among scientists 2) When you are measuring the temperature of an object what are you actually measuring? The kinetic energy or motion of the molecules 3) What unit is most accepted by scientists to measure the mass of a substance? Grams 4) What tools are used to measure the mass of a substance? Triple beam balance, balance, scale 5) Design a model to show changes in state of matter including changes in the molecular movement at each state. Include arrows and at least FOUR of the following vocabulary: condensation, deposition, evaporation, deposition, freezing, gas, liquid, solid, melting, and sublimation. Remember models include title, pics with labels, separate explanation with vocab. 6) List the components needed in an investigation. Problem, Hypothesis or Prediction, Variables, Constants, Materials, Procedures, Data, Analysis, Conclusion 7) What are procedures and why are they important? Procedures are like steps to a recipe and they provide you with the information needed to carry out the investigation 8) Why is having only one independent and dependent variable an important factor in planning an investigation? In order to prove or disprove your hypothesis and get accurate results from your investigation. 9) What process (not officially a component of an investigation) enables you to know how to set up your investigation? Research 10) A can of lemonade and a can of soda are put in the sun for 2 hours. They both came out of the fridge and had the same starting temperature, but are not at the same temperature after 2 hours. Explain why this occurred. The warmer can after 2 hours absorbed heat from the surroundings at a greater rate than the other can 11) What is the difference in an insulator and a conductor? Conductors speed up thermal energy transfer and insulators slow down thermal energy transfer 12) In the coke can lab how did we decide which was the best insulator? How could this same investigation be done but with coffee instead? In the coke can lab we tried to find the can that remained the coldest at the end of the tested period. With coffee it would work pretty much the same could you different coverings around a coffee cup such as a wool or cotton sock only you would want to see which covering kept the coffee the hottest at the end of the given time. 13) How is a line graph different than a bar graph? (Hint what do line graphs show?) A line graph shows change over time 14) What parts of a line graph need titles? X and Y axis need titles with units and the top of the graph needs a main title 15) Make a double line graph for the data below showing the circulation of daily newspapers from 1994 to 1995 grouped by Dailey and evening additions. Year Morning Evening 1994 43 15 1995 44 13 1996 40 12 1997 45 11 1998 50 10 1999 43 14 16) What is conduction? Thermal energy transfer from one substance to another by direct contact 17) What is a real life example of conduction? Pan cooking on hot stove 18) What is convection? When gases or liquids sink or rise because the cooler fluid is denser and sink 19) What is a real life example of convection? Hot air balloon 20) What is radiation? The transfer of energy in the form of electromagnetic waves 21) What is a real life example of radiation? Sun or fire 22) What is the difference in thermal expansion and contraction? When objects are heated thermal expansion can occur causing molecules to increase kinetic energy and spread out. In thermal contraction molecules slow down and move closer together. 23) Draw and describe the molecular movement of a solid, liquid, and a gas. Solid tightly packed vibrating in place, liquid moving around some slightly spread out, gas high kinetic energy spread far out 24) What happens during the process of condensation? Molecules lose KE and change from a gas to a liquid 25) What happens during the process of deposition? Molecules lose KE and change from liquid form to solid 26) What happens during the process of sublimation? Molecules gain a great amount of KE and change from a solid to a gas 27) What are constants in an investigation? Parts ot the investigation you try to keep the same. 28) Take the investigation you did with your group and either turned in or presented. Make any necessary edits to it and write out a clean copy that includes the following: a. Problem or objective b. Materials c. Independent variable d. Dependent variable e. Constants f. Procedure g. Analysis (include a blank data table)