PeriodicTable
advertisement
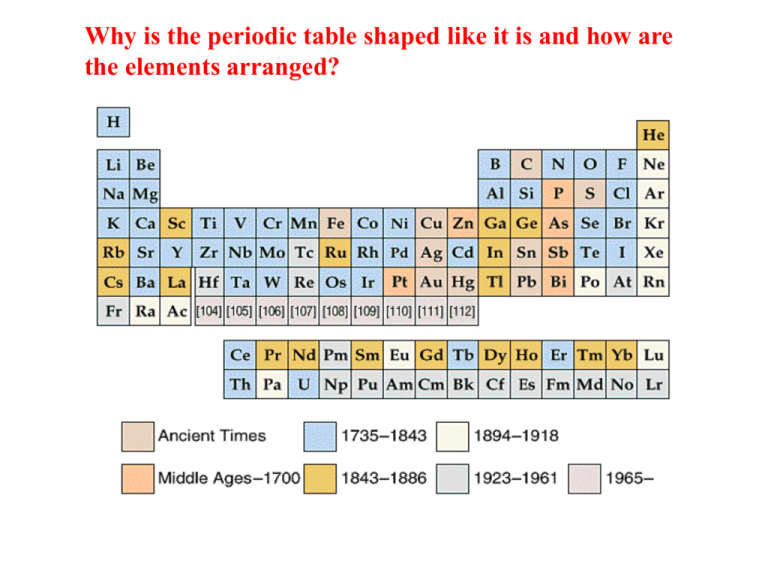
Why is the periodic table shaped like it is and how are the elements arranged? The Periodic Table - History – Two scientists, Dimitri Mendeleev (Russia) and Lothar Meyer (Germany) • properties of elements did not change smoothly with increasing atomic mas. • Instead the properties of the elements repeated periodically. – Periodic Law: the properties of the elements repeat periodically as the elements are arranged in order of increasing atomic number (# of protons) – This periodic law is used to form the Periodic Table II. The Periodic Table • John Alexander Newlands – Arranged elements in order of increasing atomic masses – Noticed some properties recurring over and over again – he called this the periodic law • Dmitri Mendeleev – Published the periodic table of elements – Very confident as he left spaces empty – assumed those elements were not yet discovered Elements are arranged: Vertically into Groups Horizontally Into Periods Groups of Elements Vertical columns on the periodic table Similar physical properties Similar chemical properties Why? Why? If you looked at one atom of every element in a group you would see… Each atom has the same number of electrons in it’s outermost shell. Valence Electrons for group A elements s block +1 ns1 +2 2 1A ns Charge on stable monatomic ion p block +3 ± 4 -3 -2 -1 8A 3A 4A 5A 6A 7A 2A Group B 105 Db 107 Bh Period number gives shell (n) 0 Elements are arranged according to atomic # and e- configuration. Li: 3 e-’s 1s2 2s1 Na: 11 e-’s 1s2 2s2 2p6 3s1 K: 19 e-’s 1s2 2s2 2p6 3s2 3p6 4s1 Paramagnetic or diamagnetic? Valence orbitals: outer shell orbitals beyond the closest noble-gas configuration Valence electrons: “the ones that can react” (located in the valence orbitals). The other e-’s are called core electrons and don’t react. 2s2 3s2 4s2 5s2 6s2 7s2 2A Be Mg Ca Sr Ba Ra Elements in a vertical row have the same number of valence electrons. 2s22p5 3s23p5 4s24p5 5s25p5 6s26p5 F Cl Br I At • The number of outer or “valence” electrons in an atom effects the way an atom bonds. • The way an atom bonds determines many properties of the element. • This is why elements within a group usually have similar properties. If you looked at an atom from each element in a period you would see… Details of the Periodic Table Period • The elements in the same horizontal row are called a period Hydrogen and Helium are in period 1 Lithium through Neon are in period 2 4 Periods on the Periodic Table 1 2 3 4 5 6 Each atom has the same number of electron holding shells. An example… The period 4 atoms each have 4 electron containing shells 4th Shell K (Potassium) Kr (Krypton) Atom Atom Fe (Iron) Atom Sublevel Blocks s1 s2 1 2 3 4 5 6 p1 p2 p3 p4 p5 p6 d1 - d10 f1 - f14 A periodic table illustrating the building-up order. Electronic Structure s block p block d block 105 Db 107 Bh f block Condensed Ground-State Electron Configurations in the First Three Periods Los Alamos National Laboratory's Chemistry Division Periodic Table of the Elements +1 +2 metals typical charge on ion atomic number in binary compound atomic mass transition metals non-metals +3 http://pearl1.lanl.gov/periodic/default.htm -3 -2 -1 noble gases Discovery dates of elements Physical States at Room Conditions gases 105 Db liquids 107 Bh Modified from http://www.cem.msu.edu/~djm/cem384/ptable.html solids Natural vs. Man-Made Elements natural elements not found on Earth 105 Db 107 Bh man-made elements Molecular Nature of Free Elements diatomic 105 Db tetratomic octatomic 107 Bh All other free elements are atomic in nature. All Isotopes Radioactive no stable forms 105 Db 107 Bh Hydride Chemistry (binary hydrogen compounds) ionic covalent polymeric salts with H- molecules with +1 chains with H-to-H bonds 1A 2A 8A 3A 4A 5A 6A 7A metallic interstitial H2 Group B 105 Db 107 Bh solids mostly gases, some liquids Important Groups Each group has distinct properties • The periodic Table is divided into several groups based on the properties of different atoms. Groups of Elements • Alkali Metals: – Group 1 metals – Soft, silver coloured metals that react violently with H2O to form basic solutions – Most reactive: cesium & francium Alkali Metals reacting with water: • • • • • Li (Lithium) Na (Sodium) K (Potassium) Rb (Rubidium) Cs (Cesium) What would you expect from Francium?!?! Chemical Periodicity Alkali metals Soft, silvery colored metals Very reactive!!! Potassium (K), in Water (H2O) Alkali Metal Family Li Na K Alkaline Earth Metals Silvery-White Metals Fairly reactive Many are found in rocks in the earth’s crust Halogens: – Group VII A, nonmetals, highly reactive. – Fluorine is the most reactive Noble Gases: -Group VIII A - Generally unreactive The Halogen Family Cl2(g) I2(s) Br2(l) Halogens Most are Poisonous Fairly reactive Chlorine Gas was used as a chemical weapon during World War I. It was used by the Nazis in World War II. Chlorine (Cl) Bromine (Br) and Iodine (I) Jellyfish lamps made with noble gases • Colors Noble Gases produce in lamp tubes(discharge tubes): Ne (Neon): orange-red • Hg (Mercury): light blue • Ar (Argon): pale lavender • He (Helium): pale peach • Kr (Krypton): pale silver • Xe (Xenon): pale, deep blue • Transition Metals: Most are good Conductors of heat and electricity Ductile and malleable (easily bent/hammered into wires or sheets) . Metalloids: Metals very close to the “staircase” line They have properties of metals and non-metals. Si (Silicon) and Ge (Germanium) are very important “semi-conductors” How many things can you think of that have Transition Metals in them? What are semiconductors used in? Nonmetals . Brittle . Do not conduct heat and electricity Modern Periodic Table Columns are called Groups or Families Elements with similar chemical and physical properties are in the same column Rows are called Periods Each period shows the pattern of properties repeated in the next period Main Group (Representative Group) Groups IA - VIIIA Transition Metals - Groups IB – VIIIB Rare Earth Elements Lanthanides (Ce - Lu) and Actinides (Th - Lr) Metals about 75% of all the elements lustrous, malleable, ductile, conduct heat and electricity Nonmetals dull, brittle, insulators Metalloids also know as semi-metals some properties of both metals & nonmetals Atomic Size Smaller S m a l l e r Li Na K Be B C N O WHY? F Ionization Energy Ionization Energy (IE) - The amount of energy needed to remove an electron from an atom or ion. Each electron in any atom or ion has a specific ionization energy. First Ionization Energy - The amount of energy needed to remove an electron from the outermost shell of a neutral (uncharged) atom. The ionization energy is the energy that must be supplied to an atom in the gas phase in order to remove an electron. If the electron is the first one to be removed, one then refers to the first ionization energy for that element. The first ionisation energy of sodium is 494 kJ.mol-1. The ionisation energy tells us how easy it is to convert an element to a cation. The lower the first ionisation energy, the easier it is to convert an element such as sodium, Na, to its cation Na+. Ionization Energy: The energy required to completely remove an e- from an atom in its gaseous state. Mg(g) Mg1+ + eMg1+(g) Mg2+ + e- 1st ionization energy 2nd ionization energy Question: Which of the above ionizations would have the highest ionization energy and why? electron being lost: 1st 2nd 3rd 4th 5th 6th 7th The first ionisation energy of most elements are known to a good degree of accuracy. It is interesting to see how these values vary with atomic number Z: The rare gases (He, Ne, Ar, Kr, Xe, Rn) appear at peak values of ionization energy, which reflect their chemical inertness, while the alkali metals (Li, Na, K, Rb, Cs) appear at minimum values of ionization energy, in keeping with their reactivity and ease of cation formation. Increases Increases Ionization energy decreases as you go down a group Ionization energy increases as you go from left to right in a period What is meant by metallic character? Periodicity of ionic radii: The size of ions differ markedly from the size of their parent atoms. Take the case of sodium as an example: The sodium atom (which has a single 3s electron), has an atomic radius of 186 pm. Upon taking up energy (the ionization energy), it loses this electron and is converted to the cation Na+, whose radius is 97 pm. The radius of cations is always smaller than the radius of the atoms from which they are derived, as shown in the figure on the right, which applies to the Group I elements: The main reason for this is that whenever metals are converted to their cations, they always do so by losing the electrons in their highest energy level. Further, since the ion has less electrons than the atom from which they are derived, there is less mutual repulsion between these electrons, and the electron orbitals shrink to some extent. What happens in the case of anions? Let's take the case of chlorine: The chlorine atom has a covalent radius of 99 pm. It can gain an extra 3p electron (with release of energy, the electron affinity) in order to form the chloride anion Cl-, with ionic radius 181 pm. The radius of anions is always larger than the covalent radius of the atoms from which they are derived, as shown in the figure on the right, which applies to the Group VII elements. The reason for this is that the additional electron increases the mutual repulsion between the electrons, and this causes an expansion of the electron orbitals. Note that the difference between the van der Waals radius of an atom and the ionic radius of its anion is not significant. Periodicity of electronegativities: When a covalent bond is formed between two identical atoms, such as H-H or Cl-Cl, the pair of electrons which joins the atoms is evenly shared between the two atoms. However, when two different atoms are joined together by a covalent bond, the sharing of the electron pair is not even, and the pair of electrons is shifted towards one of the atoms: The covalent bond is said to have been polarized, and one refers to such a bond as a polar covalent bond. The ability to polarize a covalent bond differs from one atom to another, and is known as the electronegativity of the atom. Electronegativities are measured on an arbitrary scale ranging from 0 (He) to 4.1 (F). The rare gases (He, Ne, Ar, Kr, Xe, Rn) have zero electronegativities, i.e., they only form covalent bonds, and this only in exceptional cases, and have no tendency to attract electrons of that bond. The halogens ( F, Cl, Br, I, At) appear at peak values of electronegativities. Within a period, they have the highest tendency to polarize covalent bonds. Note that within the group, the electronegativity decreases. On the whole, electronegativities increase from left to right along a period (see Li to F) and decrease from top to bottom within a group (see F to At). Periodicity of electron affinity: The electron affinity is the energy that is released when an atom in the gas phase gains an electron and is thus converted to an anion, also in the gas phase: The electron affinity of chlorine is 349 kJ.mol-1. Electron affinities are difficult to measure and there is no reliable data available for most elements. However, the larger the atom, the lower its electron affinity, as shown with Group VII elements: For reasons outside the scope of this discussion, the electron affinity of fluorine is an exception to this trend. Common Oxidation states: note the vertical similarities.







