Atomic Structure Worksheet
advertisement
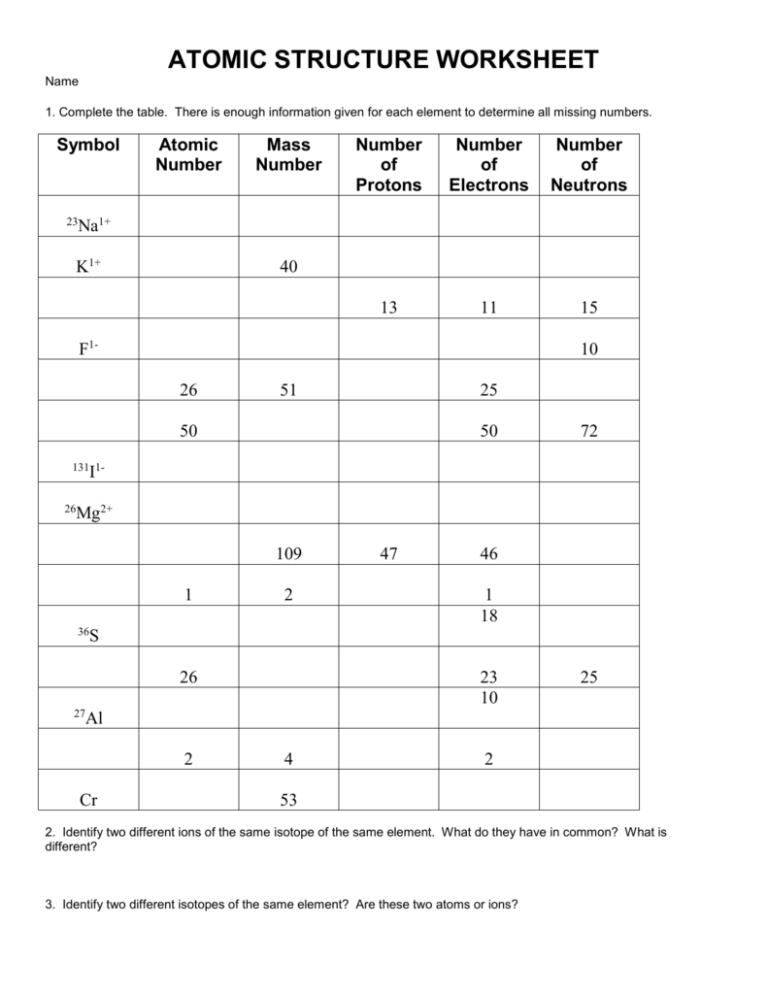
ATOMIC STRUCTURE WORKSHEET Name 1. Complete the table. There is enough information given for each element to determine all missing numbers. Symbol 23 Atomic Number Mass Number Number of Protons Number of Electrons Number of Neutrons 13 11 15 Na1+ K1+ 40 F1- 10 26 51 25 50 50 72 131 1- I 26 Mg2+ 109 1 36 2 46 1 18 S 26 27 47 23 10 25 Al 2 Cr 4 2 53 2. Identify two different ions of the same isotope of the same element. What do they have in common? What is different? 3. Identify two different isotopes of the same element? Are these two atoms or ions? 4. Draw two symbols for different ions of different isotopes of the same element? 5. Sketch a picture of a Carbon 13 (4- ion), showing the location of all subatomic particles. 6. The number of protons in one atom of an element determines the atom’s ____________________, and the number of electrons determines the _________________________ of the element. 7. The atomic number tells you the number of ___________________________ in one atom of an element. It also tells you the number of __________________________ in a neutral atom of that element. The atomic number gives the “identity” of an element as well as its location on the periodic table. No two different elements will have the ____________________ atomic number. 8. The _______________________ of an element is the total number of protons and neutrons in the ___________________ of the atom. 9. The mass number is used to calculate the number of ______________________ in one atom of an element. In order to calculate the number of neutrons you must subtract the ______________________ from the 10. If you know ONLY the following information can you ALWAYS determine what the element is? (Yes/No) a. Number of protons ____________________________ b. Number of neutrons ___________________________ c. Number of electrons in a neutral atom ___________________________ d. Number of electrons _________________________ 11. While every element is ____________________ (different or the same) from every other element…each element is made up of _____________________________ which are ________________________ (different or the same) between one element and another.