Unit 7 Quiz Study Guide – Lewis Structures, Polarity & VSEPR
advertisement
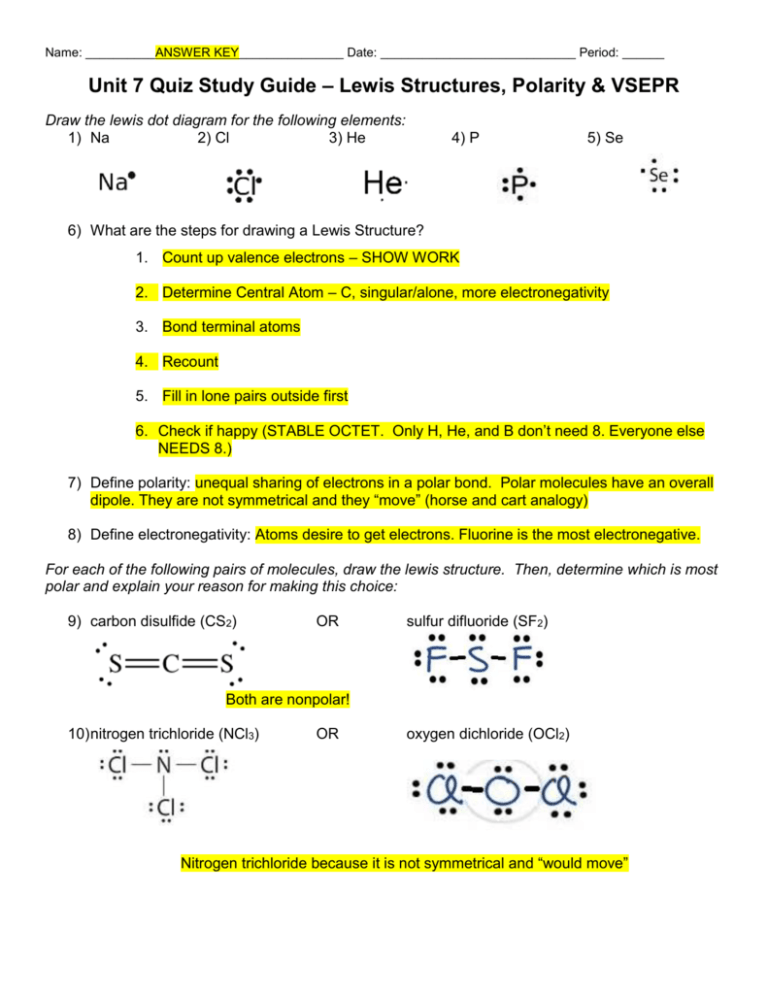
Name: __________ANSWER KEY_______________ Date: ____________________________ Period: ______ Unit 7 Quiz Study Guide – Lewis Structures, Polarity & VSEPR Draw the lewis dot diagram for the following elements: 1) Na 2) Cl 3) He 4) P 5) Se 6) What are the steps for drawing a Lewis Structure? 1. Count up valence electrons – SHOW WORK 2. Determine Central Atom – C, singular/alone, more electronegativity 3. Bond terminal atoms 4. Recount 5. Fill in lone pairs outside first 6. Check if happy (STABLE OCTET. Only H, He, and B don’t need 8. Everyone else NEEDS 8.) 7) Define polarity: unequal sharing of electrons in a polar bond. Polar molecules have an overall dipole. They are not symmetrical and they “move” (horse and cart analogy) 8) Define electronegativity: Atoms desire to get electrons. Fluorine is the most electronegative. For each of the following pairs of molecules, draw the lewis structure. Then, determine which is most polar and explain your reason for making this choice: 9) carbon disulfide (CS2) OR sulfur difluoride (SF2) Both are nonpolar! 10) nitrogen trichloride (NCl3) OR oxygen dichloride (OCl2) Nitrogen trichloride because it is not symmetrical and “would move” 11) boron trihydride (BH3) OR ammonia (NH3) Ammonia (BH3 is symmetrical – trigonal planar) 12) chlorine (Cl2) OR phosphorus trichloride (PCl3) Phosphorous trichloride is polar. Cl2 is symmetrical. 13) silicon tetrabromide (SiBr4) OR HCN HCN is polar, SiBr4 is symmetrical and therefore nonpolar. 14) Draw all of the reasonable resonance structures and the resonance hybrid for the carbonate ion, CO3 2– resonance is all the possible arrangements 15) Which element(s) can have fewer than 8 and still be stable/happy? _H__ __He_ __B__ 16) Which element can have more? _P__ 17) What does VSEPR stand for? Valence shell electron pair repulsion 18) What is VSEPR Theory used for? Determining 3D shapes of molecules 19) The molecular geometry of the H3O+ ion is _trigonal pyramid_. (Remember: Draw Lewis structure first) 20) The bond angle around the central atom in SO2 is ___. Skip this question 21) The central atom in PCl5 has __0__ lone pairs and __5_ bonded atoms. So its molecular geometry is _trigonal bipyramid_ and its bond angle is _90/120__. 22) Complete the table below Formula Lewis Structure Shape (Geometry) Polar or Nonpolar? HF Linear Polar CO2 Linear Nonpolar H2O Bent Polar CCl4 Tetrahedral Nonpolar NH4 + Tetrahedral Nonpolar 23) How many electrons are shared in a double bond? __4_ A triple bond? _6__ 24) Element X has three bonded atoms, and 0 lone pairs. What is its molecular geometry according to VSEPR? __trigonal planar____ 25) Element Y has 4 bonded atoms and 2 lone pairs. What is its molecular shape according to VSPER? __square planar___






