BIO 330 Cell Biology Lecture Outline Spring 2011 Chapter 6
advertisement

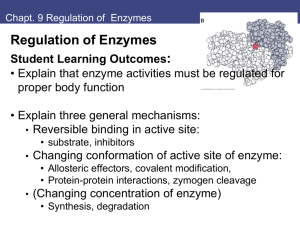
BIO 330 Cell Biology Lecture Outline Spring 2011 Chapter 6: Enzymes I. Introduction A. Bioenergetics tells us whether reactions CAN go & how much energy they release or require We need to know about mechanisms and rates of reactions, too Enzymes influence both mechanism and rate B. Definition of enzyme Protein (or RNA) catalyst for biochemical reaction II. Activation Energy and the Metastable State A. Even highly exergonic reactions do not take place quickly B. Activation energy (EA) Transition state Reaction rate is proportional to fraction of molecules with energy ≥ EA C. Metastable state Themodynamically unstable, but lack the energy to exceed EA D. Overcoming the activation energy barrier Either increase temperature OR… Lower activation energy Catalysts An agent that enhances reaction rate by lowering EA Not consumed by the reaction III. Enzymes and Biological Catalysts A. Properties of catalysts 1. Increases rxn rate by lowering EA 2. Acts by forming transient, reversible complexes with substrates 3. Changes rxn rate, not position of equilibrium B. Enzymes – specific organic catalysts Active site Prosthetic groups – nonprotein components at active site Coenzymes – small organic molecules attached to enzymes Cofactors – inorganic ions/molecules attached to enzymes Substrate specificity High specificity – enzyme will recognize only one substrate Group (low) specificity – enzyme will recognize similar substrates Enzyme diversity and nomenclature 6 classes based on general function Denaturation High temperature BIO 330 Cell Biology Lecture Outline Spring 2011 pH and general ionic strength of solution inhibitors and activators C. Enzyme activity Substrate binding Lock and key model vs induced-fit model Substrate activation Bond distortion, proton transfer, electron transfer Catalytic event Products are released following reaction IV. Enzyme Kinetics A. Reaction rates are dependent upon… Substrate concentration Enzyme concentration Product concentration Inhibitor concentration B. Michaelis-Menten kinetics Initial reaction velocity (v) Substrate concentration ([S]) Vmax = maximum velocity Saturation: a fundamental property of enzyme-catalyzed reactions V = Vmax[S] / (Km + [S]) Lineweaver-Burk double reciprocal plot C. Enzyme inhibitors Reversible vs irreversible inhibitors Irreversible inhibitors may be competitive or noncompetitive V. Enzyme Regulation A. Allosteric regulation Feedback inhibition (end-product inhibition) Allosteric enzymes exist in a high-affinity form or a low-affinity form Allosteric effectors bind enzymes at allosteric sites Shift equilibrium to high-affinity state (activator) or low-affinity state (inhibitor) In multimeric proteins: catalytic subunits vs regulatory subunits Cooperativity – increased affinity as each substrate binds B. Covalent modification Phosphorylation / dephosphorylation Kinases vs phosphatases Proteolytic cleavage