Introduction - UniMAP Portal
advertisement

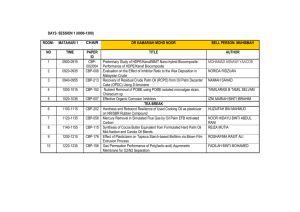
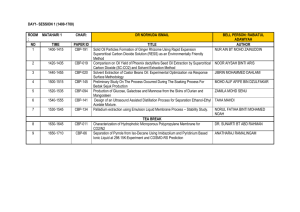
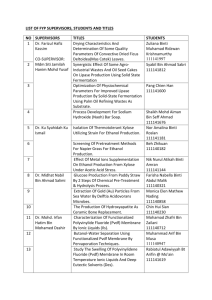
ERT 207-Analytical Chemistry Lecturers: Dr. Saleha Shamsudin (Coordinator) Assoc. Prof. Dr. Mohamed Zulkali bin Mohamed Daud Course synopsis: This course introduces and discusses the basic principle of analytical chemistry that covers data analysis and interpretation. Basic statistics and the utilization of statistics are applied in most of the analytical methods. In this course students are able to apply classical analytical methods such as gravimetry and titrimetry as well as applying modern methods in analytical chemistry such as chromatographic and spectroscopic technique. Learning Outcomes: At the end of the course, students are expected to be: 1. Ability to calculate and perform the correct statistical method for data analytical and to remember the steps in quantitative analysis. 2. Ability to classify and use separation techniques and gravimetric methods for mass determination. 3. Ability to differentiate and to calculate concentration of analytes of various titrimetric methods (acid-base, complexation, redox, and precipitation). 4. Ability to apply the chromatography principles and to interpret and calculate peak height for concentration determination. 5. Ability to understand the spectroscopic principles and to interpret and calculate concentration. List of experiments: 1.The potentiometric titration of an acid. 2.Determination of water hardness by complexometric titration. 3.Determination of limonene concentration in citrus fruit extracts by gas chromatography. 4.Interpretation of spectra of an unknown sample using Fourier transform-infrared (FTIR) spectroscopy. 5.Determination of active drug substance in a pharmaceutical formulation using ultraviolet and visible spectrometry (uv/vis). Learning approach: Lecture : 42 hours (60 %) Laboratory : 20 hours (28.6 %) Tutorial : 8 hours (11.4 %) Evaluation contribution: •Peperiksaan / Examination •Mid Term Examination 1 = 10 % •Mid Term Examination 2 = 10 % •Final Examination = 50 % : 70 % •Penilaian berterusan / Continual assessment : 30 % •Laboratories = 15 % Assignments / Quizzes = 15 % List of text books and references : Buku Teks/ Text book(s): •Gary D. Christian (2004). Analytical Chemistry. 6th Edition. Publisher: John Wiley & Sons, Inc. Buku Rujukan/ Reference books: •David Harvey (2000). Modern Analytical Chemistry. Publisher: McGraw-Hill •D. Keely and P. J. Haines (2002). Analytical Chemistry. Publisher: Oxford: Bios Scientific D. A. Skoog, D. M. West and F. J. Holler (1996). Fundamentals of Analytical Chemistry. Publisher: Saunders College Publication. Minggu/ Week Week 1 (9/09 – 13/09) Kandungan Kursus / Course Contents (Panduan/Guidelines) INTRODUCTION TO ANALYTICAL CHEMISTRY Tugasan Pensyara Bacaan/ h/Lecture Reading r Assignment Chapter 1 Dr. Saleha Week 2 – 3 UTILIZATION OF STATISTICS IN DATA ANALYSIS (16/09 – Chapter 1 Identify the significant testing. Calculate the T 27/09) test and Q test. Dr. Saleha Explain qualitative and quantitative. Repeat the steps in quantitative analysis or unit operation in analytical chemistry. BASIC STATISTICS Define accuracy and precision, remember ways of describing accuracy and precision, types of errors, understand the concept of significant figures, standard deviation. CALIBRATION CURVE Interpret slope, intercept, coefficient correlation and coefficient of determination (r2) CHEMICAL EQUILIBRIUM Discuss the concept of chemical reaction: the rate concept, type of equilibria, Le Chatelier’s principle. Effects of temperature, pressure and concentration on equilibria. Week 4 - 5 ACID-BASE EQUILIBRIUM Dr. (30/9 – Chapter 2 Saleha Repeat acids and bases theories, acid base 11/10) equilibria in water, pH scale. Discuss weak acids and bases, calculate the pH of salt of weak acids and bases. Discuss buffers. Introduce strong acids and strong bases, Apply detection of end point, indicators. Discuss weak base versus strong acid. Week 6 Cuti Pertengahan Semester / Mid-semester Break (14/10 18/10) PREPARING SAMPLES FOR ANALYSIS Discuss type of separation techniques. Week 7 - 8 Classify separation techniques based on (21/10 – chemical or physical properties. Classify 1/11) separation based on a partitioning between phases. Calculate partition coefficients and distribution ratios. Chapter 3 Dr. Saleha CLASSICAL METHODS IN TECHNIQUES OF ANALYTICAL CHEMISTRY: TITRIMETRIC METHODS OF ANALYSIS Week 9-10 Explain acid-base, redox, complexation and precipitation titrimetry. Discuss on the (4/11 – reactions involved. Calculate the 15/11) quantitative and qualitative application of acid-base, redox, complexation, precipitation titrimetry. List other application titrimetry. MODERN METHODS IN TECHNIQUES OF ANALYTICAL CHEMISTRY: CHROMATOGRAPHIC AND ELECTROPHORETIC METHODS Assoc. Prof Dr. Chapter Mohamed 4 Zulkali bin Mohamed Daud Introduce principles of chromatography and Assoc. Prof Dr. Week 11 – electrophoresis. Discuss gas chromatography and applications. Calculate quantitative and 13 Chapter Mohamed (18/11 – qualitative applications of gas 5 Zulkali bin chromatography (GC). Discuss high29/11) Mohamed performance liquid chromatography (HPLC) Daud and applications. Calculate quantitative and qualitative applications of high-performance liquid chromatography (HPLC). Discuss electrophoresis and its applications in protein and DNA analysis. MODERN METHODS IN TECHNIQUES OF ANALYTICAL CHEMISTRY: SPECTROSCOPIC TECHNIQUES Explain the properties of electromagnetic Assoc. radiation and relate with spectroscopic Prof Dr. Week 14 - techniques. Introduce techniques on Mohame spectroscopy based on absorption 15 Chapter 6 d Zulkali (ultraviolet-visible and Fourier transform(2/12 – bin infrared spectroscopy (FT-IR) and atomic 13/12) Mohame absorption spectroscopy (AAS). Demonstrate d Daud qualitative and quantitative applications of UV/visible, infrared and atomic absorption spectroscopic techniques in biological analysis. Calculate concentration using spectroscopic techniques. Week 16 (16/12– Minggu Ulangkaji / Revision Weeks 20/01) Week 17 20 Peperiksaan Akhir Semester/ Final Examination (23/12 – 10/01) LABORATORY PLAN Group 1 A1, A2 & A3 A4, A5 & A6 Hari Malaysia Exp 1 Exp 5 Exp 5 Exp 1 Exp 1 7 9 14 15 16 Exp 1 Exp 5 Exp 4 Tutorial 3 Tutorial 3 Exp 4 Exp 2 Exp 3 Tutorial 4 Tutorial 4 11 13 Tutorial 2 Tutorial 2 8 12 Exp 5 Mid Semester Break 6 10 Tutorial 1 Tutorial 1 4 5 B4, B5 & B6 Introduction to Analytical Chemistry 2 3 B1, B2 & B3 Exp 3 Exp 2 Exp 2 Exp 3 Tutorial 5 Tutorial 5 Exp 3 Tutorial 6 Lab Test Study Week Exp 2 LAB GROUPS A1 AINA MADIA BINTI RAZALI NUR HAZIRAH BINTI MOHD YUSOFF SITI NURSHAMILA BINTI ZAMZURY MOHAMAD AMIN BIN AWANG TEH @ ISMAIL LIM SHIN YUAN ANG LI WEI A2 MUHAMMAD FAIZ BIN ZAINOL BAHRI NUR AMIRAH BINTI MAHFOD SAW WAN LING NUR ASYILAH AKMAL BINTI ABDULLAH MOH LAI KUAN ANG KIAN THING B1 CHOO YEE CHING NUR AMALINA BINTI ABDULLAH SANI SITI KHADIJAH BINTI MASOR @ IBRAHIM KWAN SIN YEN ASSADUL HAQ ALI HAMIDI BOO CHIE HANG B2 NORHIDAYAH BINTI MOHAMAD NORHAIZA NADIA BINTI HALIB @ YAHAYA ONG LI JING MOHD SHAFIQ BIN MD ZIN ANNIEBELL A/P STANLEY LOURDES L.BENEDICT JASMINE FONG JIE MIN A3 B3 NURUL NABILLATUL MUNIFA BINTI R. AZMI SITI NABIHAH BINTI AB MAJID PUA CHEE WEE TAN HUAN YIH RAYMOND LIM HUNG TAT PUTRI NOOR NATASHA BINTI CHE YEO MEGAT NUR ATHIRAH AWATIF BINTI ABDUL NADYA ORSELA ANDICO RAHMAN CHUAH SIN YE LAU KIA KIAN ADZDZIN SHAFWAN BIN ADLAN CHONG WAIN KET A4 YUHANEES SYUHADAH BINTI MALIHAN NUR AMALINA BINTI MUHAMAD ZAIDI NUR AMIRAH ASIFA RAISHA BINTI ZAHARI NABILAH BINTI HUSAIN MOHAMAD ADIB BIN EDRIS CHEE CHEAU YEE WONG JING RUI NURUL NADIAH BINTI MOHAMAD NIZAM NUR SYAZWANI BINTI ISMAIL MUHAMMAD SYAFIQ BIN SANI LEONG YONG YOU CHEONG XIAN ZHI A5 VIVIAN LEE JIAQIAN ONG YU LIAN NURUL SHAKIRA BINTI JOHARI NOR SYAHIDA BINTI ISMAIL GOOI MING YAN SHARIFAH SABIRAH BINTI SYED HAMZAH B5 TENGKU INTAN NADZRAH SITI AISYAH BINTI MOHD RAMLI NUR HIDAYU BINTI MISRAN NG ZHANG JIN MUHAMAD IKRAM BIN JAMALUDIN AMIRA NASYRAH BINTI MOHD HUSIN A6 SITI RABAAH BINTI HAMZAH TAN SIEW YEN NURAINI BINTI ABDULLAH NABILAH AFIQAH BINTI SUHAIMI MUN HUI SIN B4 B6 ONG XIN SHI NURULFARAHANA BINTI HARUN NOR ATIQAH BINTI JUSRIL NG PEY XUAN ANIS USMANI Important Note: Your attendance to lecture / tutorial / laboratory is compulsory. If absent without reason, the student can not sit for the final examination.