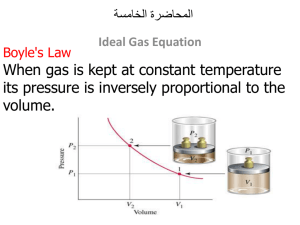
PowerPoint 簡報
advertisement
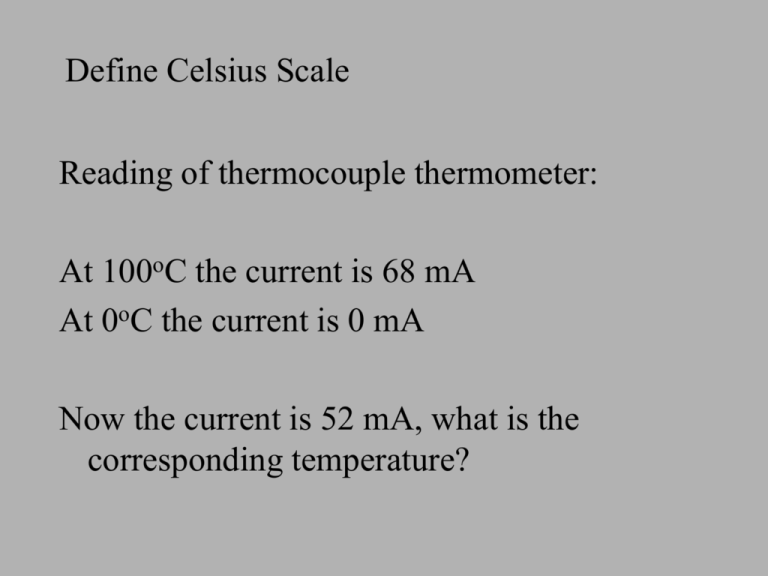
Define Celsius Scale Reading of thermocouple thermometer: At 100oC the current is 68 mA At 0oC the current is 0 mA Now the current is 52 mA, what is the corresponding temperature? Find the unknown temperature Define heat capacity C (h.c.) Define specific heat capacity (s.h.c). Explain the meaning of s.h.c. of aluminium 900 Jkg-1oC What is the heat capacity of a pot of soap if 640 000 J of energy is needed to raise its temperature from 20C to 100 C? Define power Kilowatt-hour is a unit to measure __________ 1kWh = ___________ J By experiment, the s.h.c. of water is 4200 Jkg-1C1. What does it mean? The word specific means ____________ Calculation of Energy The power of an electric heater is 2500 W. How much energy generated by this heater in half an hour? An experiment is carried out to measure the heat capacity of a copper block. The initial reading of the joulemeter is 215 100 J and the final reading is 220 830 J. The temperature of the block rises 15ºC. (a) What is the heat capacity of the block? (b) State one assumption made in the calculation. The power of a florescence tube in classroom is about 60W. There are 5 florescence tubes in classroom. How much electrical energy is used in one period of time (50 minutes) in Joule and in kilowatt-hour? If the cost of 1 kWh is about $0.6, what is the cost of electrical energy for 1 period of lesson? Mixtures When a cup of water at 20OC is mixed with another cup of water at 30OC, what is the final temperature ? 20OC 1kg + 30OC 2 kg High specific heat capacity of water s.h.c. of water = 4200Jkg-1OC-1 The high specific heat capacity of water will cause the following consequences: • it stores a large amount of ______ when it is heated up • it gives out a large amount of _______ when it cools down • it heats up or cools down more slowly than other liquids High specific heat capacity of water Living organisms • Living ____________ contain a high proportion of water • Water helps maintain a __________ body temperature when environmental temperature changes rapidly High specific heat capacity of water Climatic effects • The land heats up very fast during the day than the sea because the ______________ of soil is much less than water. • The sea cools down more slowly than the _____ during the night. • The average summer and winter temperature in ___________ vary less than inland areas. High specific heat capacity of water Water as a coolant • Materials used for cooling are called ________ • For example, water is used in a ________ to cool a car’s engine, in large air _____________ 1. How much energy is needed to heat 100g of water from 10OC to 30OC ? 2. 60000J of energy is transferred to 3kg of copper initially at 20OC. Find the final temperature of the copper. 3. A 2-kW electric heater supplies energy to a 0.5-kg copper kettle containing 1kg of water. Calculate the time taken to raise the temperature by 10OC.