In-Class Questions
advertisement

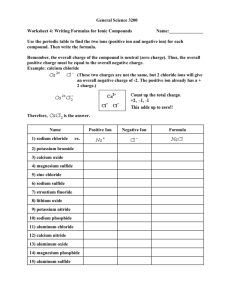
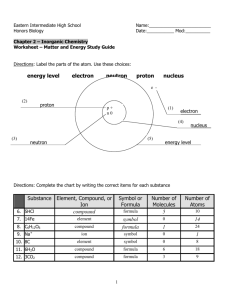
5.1 The mass ratio of lead to sulfur in lead(II) sulfide is 270.0 g lead and 41.8 g sulfur. How much lead is required to completely react with 85.6 g of sulfur? 1. 2. 3. 4. 5. 13.3 g 185 g 228 g 312 g 553 g 5.1 The mass ratio of lead to sulfur in lead(II) sulfide is 270.0 g lead and 41.8 g sulfur. How much lead is required to completely react with 85.6 g of sulfur? 1. 2. 3. 4. 5. 13.3 g 185 g 228 g 312 g 553 g 5.2 How many hydrogen atoms are in the formula ammonium phosphate, (NH4)3PO4? 1. 2. 3. 4. 5. 1 3 4 7 12 5.2 How many hydrogen atoms are in the formula ammonium phosphate, (NH4)3PO4? 1. 2. 3. 4. 5. 1 3 4 7 12 5.3 What is the carbon-hydrogen mass ratio of methane (CH4)? 1. 2. 3. 4. 5. 1 0.33 3 4 0.25 5.3 What is the carbon-hydrogen mass ratio of methane (CH4)? 1. 2. 3. 4. 5. 1 0.33 3 4 0.25 5.4 Write the chemical formula of the compound containing 2 oxygen atoms and 5 phosphorus atoms? 1. 2. 3. 4. 5. 2O5P O2P5 PO P2.5O P5O2 5.4 Write the chemical formula of the compound containing 2 oxygen atoms and 5 phosphorus atoms? 1. 2. 3. 4. 5. 2O5P O2P5 PO P2.5O P5O2 5.5 What is the total number of atoms in a molecule of glucose (C6H12O6)? 1. 2. 3. 4. 5. 2 3 6 12 24 5.5 What is the total number of atoms in a molecule of glucose (C6H12O6)? 1. 2. 3. 4. 5. 2 3 6 12 24 5.6 How many different kinds of atoms are in a molecule of penicillin (C14H20N2SO4)? 1. 2. 3. 4. 5. 41 36 14 5 1 5.6 How many different kinds of atoms are in a molecule of penicillin (C14H20N2SO4)? 1. 2. 3. 4. 5. 41 36 14 5 1 5.7 Which of these elements is a diatomic gas at room temperature? 1. 2. 3. 4. 5. Bromine Iodine Neon Chlorine Sulfur 5.7 Which of these elements is a diatomic gas at room temperature? 1. 2. 3. 4. 5. Bromine Iodine Neon Chlorine Sulfur 5.8 Which of the following species is an atomic element? 1. 2. 3. 4. 5. Mercury NaCl Oxygen gas CO2 H2 5.8 Which of the following species is an atomic element? 1. 2. 3. 4. 5. Mercury NaCl Oxygen gas CO2 H2 5.9 Aluminum carbonate is considered which of the following? 1. 2. 3. 4. 5. Atomic element Molecular element Molecular compound Ionic compound Diatomic molecule 5.9 Aluminum carbonate is considered which of the following? 1. 2. 3. 4. 5. Atomic element Molecular element Molecular compound Ionic compound Diatomic molecule 5.10 How many elements occur as diatomic molecules? 1. 2. 3. 4. 5. 4 6 3 10 7 5.10 How many elements occur as diatomic molecules? 1. 2. 3. 4. 5. 4 6 3 10 7 5.11 What is the formula of the compound formed between lithium and nitrogen? 1. 2. 3. 4. 5. LiN2 Li2N3 Li3N2 Li3N LiN 5.11 What is the formula of the compound formed between lithium and nitrogen? 1. 2. 3. 4. 5. LiN2 Li2N3 Li3N2 Li3N LiN 5.12 The name of the compound AgCl is: 1. 2. 3. 4. 5. Silver chlorine Silver chloride Silver(I) chloride Silver monochloride Monosilver monochloride 5.12 The name of the compound AgCl is: 1. 2. 3. 4. 5. Silver chlorine Silver chloride Silver(I) chloride Silver monochloride Monosilver monochloride 5.13 The name of the compound Fe2O3 is 1. 2. 3. 4. 5. Iron oxide Iron(II) oxide Iron(III) oxide Diiron trioxide Iron(II) oxide(III) 5.13 The name of the compound Fe2O3 is 1. 2. 3. 4. 5. Iron oxide Iron(II) oxide Iron(III) oxide Diiron trioxide Iron(II) oxide(III) 5.14 Identify the formulas of the following oxyanions in their correct order: sulfate ion and sulfite ion. 1. 2. 3. 4. 5. SO41– and SO31– SO31– and SO41– SO42– and SO32– SO32– and SO42– SO42– and SO3` 5.14 Identify the formulas of the following oxyanions in their correct order: sulfate ion and sulfite ion. 1. 2. 3. 4. 5. SO41– and SO31– SO31– and SO41– SO42– and SO32– SO32– and SO42– SO42– and SO3` 5.15 What is the formula of the ammonium ion? 1. 2. 3. 4. 5. NH31+ NH3 NH42+ NH4 NH41+ 5.15 What is the formula of the ammonium ion? 1. 2. 3. 4. 5. NH31+ NH3 NH42+ NH4 NH41+ 5.16 Name the following polyatomic ions in their listed order: OH1–, CO32–, and PO43–. 1. 2. 3. 4. 5. Hydroxide ion, carbonate ion, phosphate ion Oxide ion, carbonate ion, phosphate ion Hydroxide ion, carbonite ion, phosphite ion Hydroxide ion, carbonite ion, phosphate ion Oxide ion, carbonate ion, phosphite ion 5.16 Name the following polyatomic ions in their listed order: OH1–, CO32–, and PO43–. 1. 2. 3. 4. 5. Hydroxide ion, carbonate ion, phosphate ion Oxide ion, carbonate ion, phosphate ion Hydroxide ion, carbonite ion, phosphite ion Hydroxide ion, carbonite ion, phosphate ion Oxide ion, carbonate ion, phosphite ion 5.17 The name of the compound NH4ClO4 is: 1. 2. 3. 4. 5. Ammonium chloride Ammonium chlorite Ammonium hypochlorite Ammonium chlorate Ammonium perchlorate 5.17 The name of the compound NH4ClO4 is: 1. 2. 3. 4. 5. Ammonium chloride Ammonium chlorite Ammonium hypochlorite Ammonium chlorate Ammonium perchlorate 5.18 The name of the compound SnO2 is: 1. 2. 3. 4. 5. Stannium oxide Stannium(II) oxide Stannium(IV) oxide Tin(II) oxide Tin(IV) oxide 5.18 The name of the compound SnO2 is: 1. 2. 3. 4. 5. Stannium oxide Stannium(II) oxide Stannium(IV) oxide Tin(II) oxide Tin(IV) oxide 5.19 The name of the compound PI3 is: 1. 2. 3. 4. 5. Phosphorus iodide Monophosphorus triiodide Phosphorus triiodide Phosphorus(III) iodide Potassium iodide 5.19 The name of the compound PI3 is: 1. 2. 3. 4. 5. Phosphorus iodide Monophosphorus triiodide Phosphorus triiodide Phosphorus(III) iodide Potassium iodide 5.20 The name of the compound HCN(aq) is: 1. 2. 3. 4. 5. Hydrocyanic acid Hydrogen cyanide Percyanic acid Cyanic acid Hypocyanous acid 5.20 The name of the compound HCN(aq) is: 1. 2. 3. 4. 5. Hydrocyanic acid Hydrogen cyanide Percyanic acid Cyanic acid Hypocyanous acid 5.21 The name of the compound HCl(g) is: 1. 2. 3. 4. 5. Hydrochloric acid Hydrogen chloride Hypochlorous acid Chloric acid Hypochloric acid 5.21 The name of the compound HCl(g) is: 1. 2. 3. 4. 5. Hydrochloric acid Hydrogen chloride Hypochlorous acid Chloric acid Hypochloric acid 5.22 The correct formula for chloric acid is: 1. 2. 3. 4. 5. HClO HClO2 HClO3 HClO4 HClO5 5.22 The correct formula for chloric acid is: 1. 2. 3. 4. 5. HClO HClO2 HClO3 HClO4 HClO5 5.23 The correct formula for nickel(II) carbonate is: 1. 2. 3. 4. 5. NiCO3 Ni2CO3 Ni(CO3)2 NiC Ni3(CO2)2 5.23 The correct formula for nickel(II) carbonate is: 1. 2. 3. 4. 5. NiCO3 Ni2CO3 Ni(CO3)2 NiC Ni3(CO2)2 5.24 The correct formula for ammonium dichromate is: 1. 2. 3. 4. 5. NH4Cr2 NH4Cr2O4 (NH4)2Cr2O4 NH4Cr2O7 (NH4)2Cr2O7 5.24 The correct formula for ammonium dichromate is: 1. 2. 3. 4. 5. NH4Cr2 NH4Cr2O4 (NH4)2Cr2O4 NH4Cr2O7 (NH4)2Cr2O7 5.25 The correct formula for dinitrogen tetrahydride is: 1. 2. 3. 4. 5. N2H3 N2H4 N2H5 Ni2H4 N2OH4 5.25 The correct formula for dinitrogen tetrahydride is: 1. 2. 3. 4. 5. N2H3 N2H4 N2H5 Ni2H4 N2OH4 5.26 Which of the following is named correctly? 1. 2. 3. 4. 5. HF (aq), hypofluorous acid Na2HCO3, sodium bicarbonate PbO2, lead oxide SF6, sulfur heptafluoride NO2 (aq), nitrous acid 5.26 Which of the following is named correctly? 1. 2. 3. 4. 5. HF (aq), hypofluorous acid Na2HCO3, sodium bicarbonate PbO2, lead oxide SF6, sulfur heptafluoride HNO2 (aq), nitrous acid 5.27 Which of the following is named incorrectly? 1. 2. 3. 4. 5. H2SO4(aq), sulfuric acid SiO2, silicon dioxide CuCl2, copper(II) chloride Zn(OH)2, zinc(II) hydroxide NaClO, sodium hypochlorite 5.27 Which of the following is named incorrectly? 1. 2. 3. 4. 5. H2SO4(aq), sulfuric acid SiO2, silicon dioxide CuCl2, copper(II) chloride Zn(OH)2, zinc(II) hydroxide NaClO, sodium hypochlorite 5.28 What is the formula mass of sodium sulfite? 1. 2. 3. 4. 5. 215.1 amu 119.1 amu 126.05 amu 142.1 amu 125.1 amu 5.28 What is the formula mass of sodium sulfite? 1. 2. 3. 4. 5. 215.1 amu 119.1 amu 126.05 amu 142.1 amu 125.1 amu