Valency and chemical formula
advertisement

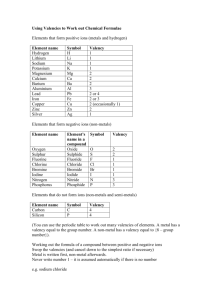
USEFUL DEFINITIONS Element A substance which cannot be split into anything simpler by chemical means. Atom The smallest part of an element that can take part in a chemical reaction. Molecule The smallest particle of a compound (a combination of two or more elements). It is also the name given to the smallest part of those elements which do not exist as atoms in the free state i.e. hydrogen nitrogen chlorine iodine H2 N2 Cl2 I2 oxygen fluorine bromine O2 F2 Br2 N.B. ionic compounds (e.g. sodium chloride) do not exist as molecules. USEFUL DEFINITIONS Ion The name given to any electrically charged atom or molecule. • positively charged ions are known as cations • negatively charged ions are known as anions Like charges repel but unlike (opposite) charges attract. If the sum of all the positive charges is equal and opposite to all the negative charges then the species will be neutral (no overall charge). Symbol A symbol represents one atom, or one mole, of an element. Valency Is a numerical measure of the combining power of an atom / ion. Historically, it was the number of hydrogen atoms which will combine with one atom, or group of atoms. It is also the number of positive (+) or negative (-) charges on an ion. e.g. Mg+2, K+, S2- Valency may also be defined as the number of electrons that an atom donates or accepts to form the duplet state (i.e., 2 electrons in outermost shell) or octet state (i.e., 8 electrons in outermost shell). The valency of an element is always a whole number. TABLE OF VALENCY valency Element/ group losing elements 1 2 All group 1 elements, hydrogen (H), Silver (Ag), ammonium (HN4) All group 2 elements, Zinc (Zn), 3 All group 3 elements 4 Carbon C), Silicon ( Si), Valencies of some elements & Radicals Valency Element/ group gaining electrons 1 All group 7 elements, hydroxide (OH), Nitrate (NO3), Hydrogen carbonate (HCO3) 2 Oxygen (O), Sulphur (S), Sulphate (SO4), Carbonate (CO3) Elements With Variable Valencies Element Symbol Valency Nomenclature Copper Cu 1, 2 Copper [I] or Cuprous Copper [II] or Cupric Silver Ag 1, 2 Silver [I] or Argentous Silver [II] or Argentic Mercury Hg 1, 2 Mercury [I] or Mercurous Mercury [II] or Mercuric Iron Fe 2, 3 Iron [II] or Ferrous Iron [III] or Ferric Tin Sn 2, 4 Tin [II] or Stannous Tin [IV] or Stannic Lead Pb 2, 4 Lead [II] or Plumbous Lead [IV] or Plumbic Phosphorus P 3, 5 Sulphur S 2, 4, 6 USEFUL DEFINITION Formula A formula represents one molecule of a compound, or the simplest ratio of the ions present. As with symbols, a formula represents a single particle or one mole of particles. The number of atoms or groups of atoms in a formula is given by putting a small number just below and behind the symbol(s). As the appearance of a symbol indicates one atom is present, a 1 isn’t written (you put NaBr not Na1Br1). In some formulae brackets are used to avoid ambiguity. Aluminium sulphate has the formula Al2(SO4)3 to show that there are two Al’s to every three SO4‘s. Without the brackets it would appear as though there were forty three O’s i.e. Al2SO43. Using valency to write chemical formulae The following steps are to be considered when writing chemical formulae • Write each symbol • Write the valency at the top right-hand side of each symbol • Cross over the valencies • Write the crossed-over valency at the bottom right of the other symbol. • Where necessary simplify the ratio of valencies Example what is the formula of aluminium oxide? Answer 1. Al O 2. Al3O2 3. Al2O3 4. Al2O3 Hence, the chemical formula of aluminium oxide is Al2O3 . Chemical formula of Calcium hydroxide 1. Ca OH 2. Ca2 OH1 3. Ca1 OH2 4. Ca(OH)2 Hence, the chemical formula of calcium hydroxide is Ca(OH)2 , chemical formula of Magnesium oxide 1. Mg O 2. Mg2 O2 3. Mg2 O2 4. Mg2 O2 5. MgO Hence, the formula of Magnesium oxide is MgO Iron (III) Sulphate 1. Fe SO4 2. Fe3SO42 3. Fe2SO43 4. Fe2(SO4) 3 The chemical formula of Iron (III) Sulphate is Fe (SO ) CONSTRUCTION OF FORMULAE It is useful to learn some formulae... Acids Gases hydrochloric acid HCl nitric acid HNO3 sulphuric acid H2SO4 ethanoic acid CH3COOH ammonia NH3 carbon dioxide CO2 methane CH4 carbon monoxide CO sulphur dioxide SO2 Write the formulae for the following compounds sodium sulphate zinc carbonate copper I nitrate Potassium chloride sulphuric acid Iron II nitrate Lead II carbonate Calcium hydroxide Magnesium sulphate Practical word equation 1. calcium carbonate + hydrochloric acid → calcium chloride + carbon dioxide + water 2. copper + silver nitrate = copper nitrate + silver 3. methane + oxygen = carbon dioxide + water 4. sulphuric acid + magnesium = magnesium sulphate + hydrogen 5. copper (II) carbonate = copper (II) oxide + carbon dioxide 6. iron (II) sulphate + sodium hydroxide = iron (II) hydroxide + sodium sulphate