File
advertisement
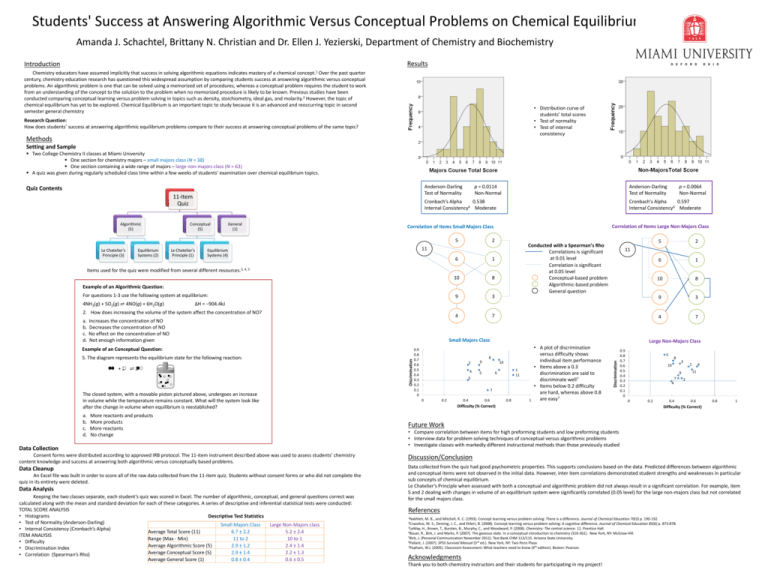
Students' Success at Answering Algorithmic Versus Conceptual Problems on Chemical Equilibrium Amanda J. Schachtel, Brittany N. Christian and Dr. Ellen J. Yezierski, Department of Chemistry and Biochemistry Results Introduction Chemistry educators have assumed implicitly that success in solving algorithmic equations indicates mastery of a chemical concept.1 Over the past quarter century, chemistry education research has questioned this widespread assumption by comparing students success at answering algorithmic versus conceptual problems. An algorithmic problem is one that can be solved using a memorized set of procedures, whereas a conceptual problem requires the student to work from an understanding of the concept to the solution to the problem when no memorized procedure is likely to be known. Previous studies have been conducted comparing conceptual learning versus problem solving in topics such as density, stoichiometry, ideal gas, and molarity.2 However, the topic of chemical equilibrium has yet to be explored. Chemical Equilibrium is an important topic to study because it is an advanced and reoccurring topic in second semester general chemistry • Distribution curve of students’ total scores • Test of normality • Test of internal consistency Research Question: How does students’ success at answering algorithmic equilibrium problems compare to their success at answering conceptual problems of the same topic? Methods Setting and Sample Two College Chemistry II classes at Miami University One section for chemistry majors – small majors class (N = 38) One section containing a wide range of majors – large non-majors class (N = 63) A quiz was given during regularly scheduled class time within a few weeks of students’ examination over chemical equilibrium topics. Anderson-Darling Test of Normality Quiz Contents 11-Item Quiz Cronbach’s Alpha 0.538 Internal Consistency6 Moderate Conceptual (5) General (1) Equilibrium Systems (2) Le Chatelier’s Principle (1) Correlation of Items Large Non-Majors Class 2 Conducted with a Spearman’s Rho Correlations is significant at 0.01 level Correlation is significant at 0.05 level Conceptual-based problem Algorithmic-based problem General question 11 Equilibrium Systems (4) 6 1 10 8 9 3 4 7 Items used for the quiz were modified from several different resources.3, 4, 5 Example of an Algorithmic Question: For questions 1-3 use the following system at equilibrium: 4NH3(g) + 5O2(g) ⇌ 4NO(g) + 6H2O(g) Increases the concentration of NO Decreases the concentration of NO No effect on the concentration of NO Not enough information given 2 6 1 10 8 9 3 4 7 11 Small Majors Class 5. The diagram represents the equilibrium state for the following reaction: +2 ⇌2 The closed system, with a movable piston pictured above, undergoes an increase in volume while the temperature remains constant. What will the system look like after the change in volume when equilibrium is reestablished? More reactants and products More products More reactants No change Data Collection Consent forms were distributed according to approved IRB protocol. The 11-item instrument described above was used to assess students’ chemistry content knowledge and success at answering both algorithmic versus conceptually based problems. Data Cleanup An Excel file was built in order to score all of the raw data collected from the 11-item quiz. Students without consent forms or who did not complete the quiz in its entirety were deleted. Data Analysis Keeping the two classes separate, each student’s quiz was scored in Excel. The number of algorithmic, conceptual, and general questions correct was calculated along with the mean and standard deviation for each of these categories. A series of descriptive and inferential statistical tests were conducted: TOTAL SCORE ANALYSIS • Histograms Descriptive Test Statistics • Test of Normality (Anderson-Darling) Small Majors Class Large Non-Majors class • Internal Consistency (Cronbach’s Alpha) Average Total Score (11) 6.7 ± 2.2 5.2 ± 2.4 ITEM ANALYSIS Range (Max - Min) 11 to 2 10 to 1 • Difficulty Average Algorithmic Score (5) 2.9 ± 1.2 2.4 ± 1.4 • Discrimination Index Average Conceptual Score (5) 2.9 ± 1.4 2.2 ± 1.3 • Correlation (Spearman’s Rho) Average General Score (1) 0.8 ± 0.4 0.6 ± 0.5 Discrimination Example of an Conceptual Question: a. b. c. d. 5 ∆H = −904.4kJ 2. How does increasing the volume of the system affect the concentration of NO? a. b. c. d. p = 0.0064 Non-Normal Cronbach’s Alpha 0.597 Internal Consistency6 Moderate Correlation of Items Small Majors Class 5 Le Chatelier’s Principle (3) Anderson-Darling Test of Normality 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 Large Non-Majors Class 8 2 4 9 10 1 11 6 5 3 7 0 0.2 0.4 0.6 0.8 1 • A plot of discrimination versus difficulty shows individual item performance • Items above a 0.3 discrimination are said to discriminate well7 • Items below 0.2 difficulty are hard, whereas above 0.8 are easy7 Discrimination Algorithmic (5) p = 0.0114 Non-Normal Difficulty (% Correct) 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 5 8 10 3 1 11 9 7 4 0 0.2 0.4 6 2 0.6 0.8 1 Difficulty (% Correct) Future Work • Compare correlation between items for high preforming students and low preforming students • Interview data for problem solving techniques of conceptual versus algorithmic problems • Investigate classes with markedly different instructional methods than those previously studied Discussion/Conclusion Data collected from the quiz had good psychometric properties. This supports conclusions based on the data. Predicted differences between algorithmic and conceptual items were not observed in the initial data. However, inter item correlations demonstrated student strengths and weaknesses in particular sub concepts of chemical equilibrium. Le Chatelier’s Principle when assessed with both a conceptual and algorithmic problem did not always result in a significant correlation. For example, item 5 and 2 dealing with changes in volume of an equilibrium system were significantly correlated (0.05 level) for the large non-majors class but not correlated for the small majors class. References 1Nakhleh, M. B., and Mitchell, R. C. (1993). Concept learning versus problem solving: There is a difference. Journal of Chemical Education 70(3) p. 190-192. 2Cracolice, M. S., Deming, J. C., and Ehlert, B. (2008). Concept learning versus problem solving: A cognitive difference. Journal of Chemical Education 85(6) p. 873-878. 3LeMay, H., Brown, T., Bursten, B., Murphy, C., and Woodward, P. (2008). Chemistry: The central science. 11. Prentice Hall. 4Bauer, R., Birk, J. and Marks, P. (2007). The gaseous state. In a conceptual introduction to chemistry (316-361). New York, NY: McGraw-Hill. 5Birk, J. (Personal Communication November 2011). Test Bank CHM 113/115. Arizona State University. 6Pallant, J. (2007). SPSS Survival Manual (3rd ed.). New York, NY: Two Penn Plaza. 7Popham, W.J. (2005). Classroom Assessment: What teachers need to know (4th edition). Boston: Pearson. Acknowledgments Thank you to both chemistry instructors and their students for participating in my project!






