3 Weeks Test Review

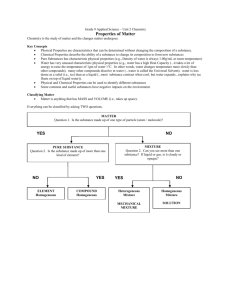
Which sample of matter is classified as a pure substance?
(A) Air (B) milk (C) ammonia (D) seawater
Air = homogeneous mixture of oxygen(~80%) and nitrogen (~20%)
Ammonia NH3 (compounds are pure substances)
Milk= cream and water (homogeneous mixture)
Seawater= salt and water (homogeneous mixture)
Homogeneous Mixtures also called SOLUTIONS.
Which statement describes a chemical change?
A. Alcohol evaporates. Evaporation physical change
C. Table salt (NaCl) is crushed into powder.
D. Glucose (C
6
H
12
O
6
) and oxygen produce CO
2 and H
2
O
Substance reacts to produce new substances
A mixture of sand and table salt can be separated by filtration because the substances in the mixture differ in:
(A) boiling point
(C)freezing point
(B) density at STP
(D) solubility in water
Which substance can not be broken down by a chemical change?
(A) ammonia (B) mercury (C) propane (D) water
NH3 Hg C
3
H
8
H2O
Which statement describes a chemical property of silicon?
(A) Silicon has a blue-gray color.
(B) Silicon is a brittle solid at 20°C.
(C) Silicon melts at 1414°C.
(D) Silicon reacts with fluorine.
During the Gold-rush, miners used density to distinguish gold form pyrite. Gold, an element, has a density of 19 g/cm 3 and pyrite (also known as “fools gold”) is a mineral with a density of 3 g/cm 3 . The property “density” is:
(A) Chemical and Intensive
(B) Chemical and Extensive
(C) Physical and Intensive
(D) Physical and Extensive
Density is just mass divided by volume. We can measure mass, we can measure volume, therefore it’s a physical property. Also, density will not vary as you vary the amount of your substance.
Remember: A cup of water has a density of 1 g/mL just as a swimming pool full of water also has a density of
1 g/mL.
Several pieces of laboratory glassware are available for student use, including beakers, Erlenmeyer flasks, pipettes, graduated cylinders, and volumetric flasks. Which of the following sets contain only glassware used to measure volume with the greatest accuracy?
(A) Graduated cylinders and volumetric flasks
(B) Beakers and graduated cylinders
(C) Erlenmeyerlasks and volumetric flasks
(D) Pipettes and beakers
Use the following passage to answer questions 8 and 9:
Iron has been used for thousands of years. In the air, iron corrodes to form rust. One reaction for the corrosion of iron is represented by the balanced equation:
4Fe(s) + 3O
2
( g ) → 2Fe
2
O
3
(s)
8) Identify one substance in the passage that can not be broken down by a chemical change.
9) Which substance is a gas at room temperature.
Using grammatically correct sentences, answer questions 10 and 11.
10) Explain the difference between a chemical property of matter and a physical property of matter.
A chemical property is a property of matter that you can observe or measure and in the process you might create a new substance.
(ex. Flammability, reactivity, and ability to rust /corrode
/tarnish)
A physical property is something you can observe or measure without changing the identity of your sample of matter. (ex.
Color, density, mass, melting point, boiling point)
Therefore the difference is that when you are observing or measuring a chemical property you might change the identity and when you measure or observe a physical property you do not change the identity.
PROPERTY
Using grammatically correct sentences, answer questions 10 and 11.
11) Use ALL of the terms properties of matter, extensive,
intensive, and help identify, to explain the similarities between mass and melting point.
Sample:
Both mass and melting point are properties of matter, but they differ in the fact that mass is an extensive property because it will change if you change the size of your sample and melting point is an intensive property because melting point will not change if you were to change the size of your sample.
A student prepared two mixtures, each in a labeled beaker.
Enough water at 20°C was used to make 100 milliliters of each mixture.
12) Using a complete sentence, classify each mixture using the term “homogeneous” or the term “heterogeneous.”
Mixture 1 (NaCl in H2O) is homogeneous because it has no visible parts; on the other hand, mixture 2 is heterogeneous because you can see a black solid on the bottom of the beaker.
13) Determine the volume of the Fe filings used to produce mixture.
Report your answer using the correct number of significant figures.
Density= mass / volume , when you solve for volume you get:
Volume = mass/density So,
Volume = 15.9 g / 7.87 g/cm 3 = 2.0203303 cm 3
But that’s the calculator answer, looking at the rules for sig figs we know that we have to round to the least number of sig. figs. because we are dividing, and that’s 3 sig.figs. Therefore, our answer is 2.02 cm 3
14) Describe a procedure to physically remove the water from mixture 1
Filtering will not work, the salt is dissolved in the water and will go through the filter paper like it did in our lab.
Therefore, we must evaporate the water and the salt crystals will remain in the beaker because salt has a higher boiling point than water .