Ch. 2-1 Nature of Matter
advertisement

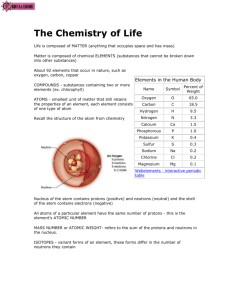
The Chemistry of Life: The Nature of Matter Biology Ch. 2 Ms. Haut Life’s Hierarchical Order • • • • • • • • • • • Atom Molecule Organelle Cell Tissue Organ Organ system Organism Population Community Ecosystem Matter is Made of Atoms • Atom—smallest unit of matter that cannot be broken down by chemical means • Made up of subatomic particles – protons – neutrons – electrons Elements Are the Simplest Pure Substances • Elements—substance made of only one kind of atom – Elements are represented by a one- or twoletter symbol. Elements • The number of protons in an atom of an element is the element's atomic number. • Is also the number of electrons • Atomic weight (mass) is equal to the number of protons and neutrons Isotopes • Atoms of an element that contain a different number of neutrons • Identified by their mass numbers http://www.uwm.edu/Dept/EHSRM/RAD/Figure_1.jpg Elements and Isotopes • Radioactive Isotopes – Their nuclei are unstable and break down at a constant rate over time, releasing energy – Although the radiation these isotopes give off can be dangerous, they have important scientific and practical uses. Elements and Isotopes • Radioactive isotopes can be used: • • • • to determine the ages of rocks and fossils. to treat cancer. to kill bacteria that cause food to spoil. as labels or “tracers” to follow the movement of substances within an organism. BIOLOGY: CONCEPTS AND CONNECTIONS 4th Edition, by Campbell, Reece, Mitchell, and Taylor, ©2003 Atoms Can Bond Together • Chemical compound—substance made of 2 or more different elements • Emergent properties—new properties present at one level that are not seen in the previous level sodium + chlorine sodium chloride Atoms Can Bond Together • Compounds linked together by chemical bonds Bond Strength – Covalent • Non-polar • Polar – Ionic – Hydrogen – Van der Waals interactions Covalent Bonding • Valence electrons are shared by atoms • Sharing electrons means that the moving electrons actually travel in the orbitals of both atoms. Covalent Bonds • Electronegativity—the attraction for electrons • Nonpolar Covalent – Electrons are shared evenly • Polar Covalent – One atom more electronegative than the other (charged) – water Hydrogen Bonding • Hydrogen atom covalently bonded to one electronegative atom is attracted to another electronegative atom (oxygen or nitrogen) • High electronegativity difference strips valence electrons away from another atom • Electron transfer creates ions (charged atoms) – Cation (positive ion) – Anion (negative ion) Na+ Cl– BIOLOGY: CONCEPTS AND CONNECTIONS 4th Edition, by Campbell, Reece, Mitchell, and Taylor, ©2003 http://www.sacbee.com/static/weblogs /health-andfitness/Salt%2520Shaker.jpg Ionic Bonding Van der Waals Forces van der Waals forces form between the molecules on the surface of a gecko’s foot and the molecules on the surface of the wall, allowing it to grip the wall • When molecules are close together, a slight attraction can develop between the oppositely charged regions of nearby molecules. • Not as strong as ionic bonds or covalent bonds, they can hold large molecules together The Nature of Matter The Nature of Matter The particles that move around the nucleus of an atom are called a) b) c) d) neutrons. protons. electrons. isotopes. The Nature of Matter The atomic number of a carbon atom is 6. How many neutrons does the isotope carbon-14 have? a) b) c) d) 6 8 12 14 The Nature of Matter Which of the following statements about the three isotopes of carbon is true? a) They are all radioactive. b) They have different numbers of electrons. c) They have the same chemical properties but differ in atomic mass. d) They have the same number of protons and neutrons. The Nature of Matter A chemical compound consists of a) Electrons mixed with neutrons. b) two or more elements combined in a definite proportion. c) two or more elements combined in any proportion. d) at least three elements combined by ionic or covalent bonds. The Nature of Matter Van der Waals forces are the result of a) b) c) d) unequal sharing of electrons. ionic bonds. the bonding of different isotopes. the chemical combination of sodium and chlorine. References • Unless otherwise noted, illustrations are credited to Prentice Hall and have been borrowed from Biology by Miller and Levine, © 2007. These images have been produced from the originals by permission of the publisher. These illustrations may not be reproduced in any format for any purpose without express written permission from the publisher.