Risky Business: Understanding Risk and the Application of Number

Risky Business: Understanding
Risk and the Application of
Number-Needed-to-Treat
Patti Ragan PhD, MPH, PA-C
Brenda Quincy PhD, MPH, PA-C
AAPA San Francisco, CA.
May 24, 2016
1
Session Objectives
• At the end of this session, participants will be able to:
1.
Define, compare and contrast the concepts of: absolute risk reduction (or increase), relative risk reduction
(RRR), number-needed-to-treat (NNT) and numberneeded-to-harm (NNH)
2.
Discuss how NNT, NNH, and NNS (number-needed-toscreen) provide tools to translate the outcomes of large clinical studies to individual patient situations
3.
Calculate NNT, NNH and NNS from raw data and study outcomes
4.
Identify published NNT resources
2
It’s the Information Age…
•
So, what is the problem???
•
The question is raised…
• How do the findings from randomized controlled trials or systematic review and meta-analyses apply to individual patients??
3
Application Challenges
• The risk of an individual may not be similar to the
“average risk” of the group studied
• In a study with a small sample size, subgroup analyses must be conducted and interpreted cautiously
• Effective treatments or preventive measures from a large study may not translate to the same benefit in an individual patient
• The relative risk or odds ratio for a given outcome may difficult to conceptualize for an individual patient
4
How Can We Apply Results to Our
Patients?
• An outcome measure with greater clinical utility than the relative risk (RR) or odds ratio (OR) is the number-needed-to-treat (NNT) or the numberneeded-to-harm (NNH)
• NNT – the number of people who must undergo the proposed intervention for one person to experience benefit
• NNH – the number of people who must undergo the proposed intervention for one additional person to experience harm
5
Other Expressions of Risk
• Absolute risk reduction
• Relative risk reduction
6
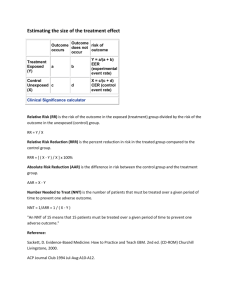
Absolute Risk Reduction
• Absolute risk reduction (ARR) is the difference in risk between treatment (experimental) and control groups
• Calculate the risk in the experimental group
# in treatment group who experience the outcome
Total number in treatment group
• Calculate the risk in the control group
# in control group who experience the outcome
Total number in control group
• Subtract the risk in the experimental group from the risk in the control group
7
Relative Risk Reduction
• Let’s start with relative risk…
• RR = risk in treatment group/risk in control group
• RR > 1 means risk is higher with treatment
• RR < 1 means treatment is protective
• RR = 1 means the risk is the same for both groups
• RRR- the amount by which the risk in the treatment group is reduced relative to the controls
•
• RRR = (risk in controls – risk in treatment) risk in controls
8
An Example
• Hypothetical example
• 100 women take a new drug (treatment) to see if it reduces the incidence of breast cancer vs. 100 women who take a placebo (control). After 5 years of follow-up,
2 women in the treatment group get breast cancer, compared to 4 in the control group.
• 100 women in treatment group followed 5 years → 2 develop breast cancer
• 100 women in control group followed 5 years → 4 develop breast cancer
Source credit: Extrapolated from Cuzick, Powles, Veronesi, Forbes, et al. Overview of the main outcomes in breast cancer prevention trials. The Lancet. Vol.361 Jan 25, 2003
9
Absolute Risk Reduction
• ARR = risk in control group – risk in treatment group
• Risk (controls) = # breast cancers in control group total # in control group
= 4/100 = .04 or 4%
• Risk (tx group) = # breast cancers in treatment group total # in treatment group
= 2/100 = .02 or 2%
• ARR = 4% - 2% = 2% reduction in risk
10
Relative Risk Reduction
• RRR = risk reduction with new treatment relative to control
• RRR = (risk in controls – risk in treatment group) risk in controls
= .04 - .02
.04
= 0.5 50% relative risk reduction
11
What is the Difference?
• Can you see the headline????
• New drug reduces breast cancer risk by 2%
Or
• In a newly released study, women taking a new breast cancer prevention drug had a relative reduction in their breast cancer risk of 50%!
12
3
2.5
2
1.5
1
0.5
4.5
4
3.5
0
ARR versus RRR
Absolute Risk
Reduc on –
4 /100 - 2 /100 =
2 /100 = 2% reduc on
Rela ve Risk
Reduc on –
(4/100 - 2 /100)/
(4/100) = 2/4 =
50% reduc on
Control
Experimental
ARR RRR
13
Back to Clinical Utility
• Is there a more clinically useful measure of the effects of a new treatment on risk than the ARR and
RRR?
• Number-Needed-to-Treat (NNT)
• The number of people who need to receive a new therapy for one more person to benefit (have a good outcome)
• NNT = 1
ARR
14
Caveats for Applying NNT
• There needs to be a sense of follow-up time
• How long do you have to treat to obtain the good outcome or prevent the bad outcome?
• NNT is an estimate and should be viewed with confidence intervals. especially in a small study
• The smaller the NNT, the better.
• NNT= 1 means every person benefits
15
NNT
• NNT = 1/ARR
• ARR = the amount of risk reduction for those receiving the treatment
• Example
• If a new drug reduces risk of a MI from 50% to 30%
• ARR = 0.5 – 0.3 = 0.2
• NNT = 1/0.2 = 5
• Interpretation – 5 people need to be treated with the new drug to prevent a MI in one person
Number-Needed-to-Harm (NNH)
• NNH – the number of people who need to be treated for one more person to experience harm
• NNH = 1/(absolute risk increase ARI)
• ARI = risk of bad outcome in tx group minus risk of bad outcome in control group
• Example
• If a drug increases the risk of a bad outcome (adverse effect) from 10% to 40%
• ARI = 0.4 – 0.1 = 0.3 (30%)
• NNH = 1/0.3 = 3
• Interpretation: 3 people need to be treated for 1 more person to experience the bad outcome (adverse effect)
17
NNT Example
• Aim – to determine the effect on mortality of giving thrombolytic therapy in the treatment of acute MI
• If mortality is 12% in the control group and 9% in the thrombolytic therapy group:
•
• ARR = 0.12 – 0.09 = 0.03 (3%) then
• NNT = 1/ARR = 1/0.03 = 33
• Interpretation: In the setting of an acute MI, 33 patients would need to be treated with thrombolytic therapy to save one additional life
18
The “Risks” of Benefits
• Giving thrombolytic therapy also increases the risk of intracranial hemorrhage. NNH can help us evaluate the potential risk…
• If the absolute risk increase is 1%, then
• NNH = 1/0.01 = 100
• Interpretation: 100 patients would need to be given thrombolytic therapy to cause one fatal intracranial hemorrhage
19
Terminology Definitionsaquations
Absolute risk reduction (ARR) is the amount by which therapy reduces the risk of a bad outcome.
Absolute Risk Reduction (ARR)
ARR = CER (control event rate) – EER (experimental event rate)
Relative Risk Reduction (RRR)
Relative risk reduction (RRR) is the ratio of the absolute risk reduction (ARR) divided by the control event rate (CER).
RRR = CER - EER
CER
Absolute risk increase (ARI) is the amount by which therapy increases the risk of one additional bad outcome.
Absolute Risk Increase (ARI)
Number Needed to Treat (NNT)
ARI = EER (experimental event rate) – CER (control event rate)
Number needed to treat (NNT) is the number of patients that need to be treated for one to receive benefit. It can also represent the number of patients that need to be treated to prevent one additional bad outcome.
NNT*= 1/ARR
Number Needed to Harm
(NNH)
Number needed to harm (NNH) is the number of patients that need to be treated for one additional bad outcome to occur (harm).
NNH* = 1/ARI
20
* If using percentage instead of proportion with ARR or ARI, divide into 100 instead of 1.
1
Key points for NNTs
Number-needed-to-treat…..
• Can be used to evaluate benefit and risk for an individual patient, based on his/her values and preferences
• Can be calculated from raw data, OR, RRR and expected prevalence
• Is typically calculated for binary (dichotomous) outcomes – time-to-event outcomes require more complex calculations
• Is an estimate of effect size (should include a confidence interval as a reflection of precision)
• Should only be calculated from comparable studies with clinical homogeneity
• Should include the duration of treatment for the given effect
• Is more certain when calculated from rigorous systematic reviews or meta-analyses
• Is best when it is a small number (small number need treatment to see benefit)
• Is only applicable if current patient has similar characteristic to those in the study from which NNT is derived
21
Time for Application
• Case 1: A 54 year-old male presents to the emergency room with complaints of pleuritic chest pain that seems to be worse when lying supine. His past medical history is significant for the placement of a LAD stent following acute myocardial infarction 8 weeks ago. He has been faithfully attending cardiac rehab and compliant with his medications. The pain started this morning with no clear precipitating factors.
22
Additional History
• He denies shortness of breath, orthopnea, PND or pedal edema. The pain worsens with deep breathing so he has tried to breathe more shallowly. He reports no palpitations. This does not feel like the pain he had with his heart attack. He tried nitroglycerin and it did not help.
He denies fever, chills, cough or congestion.
• Current medications: metoprolol 100mg po BID, lisinopril 10mg po BID, clopidogrel 75mg po q day, aspirin 81 mg po q day, atorvastatin 80 mg po q day; nitroglycerin 0.4mg SL q 5 min x 3 prn chest pain
• Allergies: NKDA
23
Physical Exam
General assessment: Well developed, well nourished male appears moderately uncomfortable.
Vitals: BP 132/86; HR 102 bpm, regular; RR 20; Ht
5’10”, Wt 180lbs; O
2 sat by pulse oximetry 97%
Lungs – clear to A&P
Heart – heart tones distant; regular rate and rhythm without m/g/r; questionable pericardial friction rub
Abd - +BS, soft, nontender
24
Labs
• CBC and Chem panel within reference range
• Cardiac enzymes – within reference range
• ESR & CRP – both elevated
• Chest x-ray – mild cardiac enlargement
• EKG – diffuse concave upward ST segment elevation with PR segment depression in II, AvF and V4-V6.
• Transthoracic echocardiogram – pericardial effusion
25
Diagnosis & Management
• What is the most likely diagnosis?
• Dressler’s syndrome
• What is the current standard of care for management for this patient?
26
A Randomized Trial of Colchicine for Acute Pericarditis
Background
Colchicine is effective for the treatment of recurrent pericarditis. However, conclusive data are lacking regarding the use of colchicine during a first attack of acute pericarditis and in the prevention of recurrent symptoms.
Methods
In a multicenter, double-blind trial, eligible adults with acute pericarditis were randomly assigned to receive either colchicine (at a dose of 0.5 mg twice daily for
3 months for patients weighing >70 kg or 0.5 mg once daily for patients weighing
≤70 kg) or placebo in addition to conventional antiinflammatory therapy with aspirin or ibuprofen. The primary study outcome was incessant or recurrent pericarditis.
27
A Randomized Trial of Colchicine for Acute Pericarditis
Results: A total of 240 patients were enrolled, and 120 were randomly assigned to each of the two study groups. The primary outcome occurred in 20 patients ( 16.7% ) in the colchicine group and 45 patients ( 37.5% ) in the placebo group ( relative risk reduction in the colchicine group , 0.56
; 95% confidence interval, 0.30 to 0.72; number needed to treat, 4 ; P<0.001). Colchicine reduced the rate of symptom persistence at 72 hours (19.2% vs. 40.0%, P = 0.001), the number of recurrences per patient (0.21 vs. 0.52, P = 0.001), and the hospitalization rate (5.0% vs. 14.2%, P =
0.02). Colchicine also improved the remission rate at 1 week (85.0% vs. 58.3%,
P<0.001). Overall adverse effects and rates of study-drug discontinuation were similar in the two study groups. No serious adverse events were observed.
Conclusions: In patients with acute pericarditis, colchicine, when added to conventional antiinflammatory therapy, significantly reduced the rate of incessant or recurrent pericarditis.
28
Colchicine and Acute Pericarditis
• Describe the intervention and the primary outcome in this study.
• Intervention is colchicine or placebo + NSAIDs.
• Primary outcome is incessant or recurrent pericarditis
29
ARR
• What is the absolute risk reduction for the primary outcome?
• Risk (incidence) colchicine = 20/120 = 0.1667
• Risk (incidence) placebo = 45/120 = 0.375
• Absolute risk reduction = 0.375 – 0.1667 = 0.2083
• What does this mean?
• The risk of incessant or recurrent pericarditis is 20% lower for those taking colchicine in addition to usual care compared with usual care alone
30
NNT
• Calculate the NNT for the primary outcome.
• NNT = 1/0.2083 = 4.8 = 4
• Explain what the result means.
• 4 people need to be treated with colchicine and traditional
NSAID to prevent one case of incessant or recurrent
pericarditis.
Note: always go conservative – round down for NNT and up for NNH
31
Case 2
• L.S. is a 49 year-old female who presents for her annual well woman exam including a Pap test and mammogram. Recently she read something about ovarian cancer risk being higher in nulliparous women.
She has never been pregnant and remembered that one of her mother’s 4 sisters had ovarian cancer and she was the only one of them who had not had children.
L.S. wonders if she should have “the ovarian cancer test.”
32
Questions, Questions
• What are the risk factors for ovarian cancer?
•
What are the symptoms of ovarian cancer?
•
What tests are available to screen for ovarian cancer?
33
Ovarian CA Screening Guidelines
• USPSTF – recommends against screening for ovarian cancer in asymptomatic women without known gene mutations (Grade D)
• Why? Low incidence, low PPV, high FPs, no evidence that screening decreases mortality
• ACOG and NIH Consensus Panel also recommend against screening
• ACOG recommends annual gynecologic exam
34
Purpose of Screening
• To identify disease for which there is an effective therapy early enough for the treatment to positively impact the outcome
35
Criteria for a Good Screening Program
• Disease state
• Early detection and early treatment leads to better outcomes
• Major cause of death and substantial prevalence
• Treatments and facilities should be available
• Test
• Simple
• Acceptable & safe
• Cost effective
• Accurate (high sensitivity, specificity, PPV, NPV, LR)
36
WHO criteria
Review of Test Characteristics
• Sensitivity
• Ability of the test to capture a true positive
• Probability of a positive test result in those with disease
• Specificity
• Ability of test to capture a true negative
• Probability of a negative test in those without disease
• Positive predictive value
• Probability of disease in those with a positive test
• Negative predictive value
• Probability of no disease in those with a negative test
• Likelihood ratio
• Probability of a test result in people with disease divided by the probability of a test result in people without the disease
37
Two Potential Biases
• Lead time bias
• Definition – when a disease is detected prior to symptom development but the treatment does not make the patient live longer; it may look like life expectancy increased but really it was simply detected earlier
• Length bias
• Diseases with a more insidious onset tend to have a longer pre-symptomatic stage so are more likely to be detected in a screening program, again appearing to lengthen survival time
38
39
Lead Time
Bias
Length Bias
40
Back to Our Patient…
• 49 years old
• Nulliparous
• Maternal aunt with ovarian cancer
Do we recommend screening?
41
Effect of Screening on Ovarian Cancer Mortality
The Prostate, Lung, Colorectal and Ovarian (PLCO)
Cancer Screening Randomized Controlled Trial
Context: Screening for ovarian cancer with cancer antigen 125 (CA-
125) and transvaginal ultrasound has an unknown effect on mortality.
Objective To evaluate the effect of screening for ovarian cancer on mortality in the Prostate, Lung, Colorectal and Ovarian (PLCO)
Cancer Screening Trial.
Design, Setting, and Participants Randomized controlled trial of
78,216 women aged 55 to 74 years assigned to undergo either annual screening (n=39,105) or usual care (n=39,111) at 10 screening centers across the United States between November 1993 and July 2001.
42
Effect of Screening on Ovarian Cancer Mortality
The Prostate, Lung, Colorectal and Ovarian (PLCO)
Cancer Screening Randomized Controlled Trial
Intervention The intervention group was offered annual screening with CA-125 for 6 years and transvaginal ultrasound for 4 years. Participants and their health care practitioners received the screening test results and managed evaluation of abnormal results. The usual care group was not offered annual screening with CA-125 for 6 years or transvaginal ultrasound but received their usual medical care. Participants were followed up for a maximum of 13 years (median [range], 12.4 years [10.9-13.0 years]) for cancer diagnoses and death until
February 28, 2010.
43
Effect of Screening on Ovarian Cancer Mortality
The Prostate, Lung, Colorectal and Ovarian (PLCO)
Cancer Screening Randomized Controlled Trial
Main Outcome Measures Mortality from ovarian cancer, including primary peritoneal and fallopian tube cancers. Secondary outcomes included ovarian cancer incidence and complications associated with screening examinations and diagnostic procedures.
Results Ovarian cancer was diagnosed in 212 women (5.7 per 10 000 person-years) in the intervention group and 176 (4.7 per 10000 person-years) in the usual care group ( rate ratio [RR], 1.21
; 95% confidence interval [CI], 0.99-1.48). There were
118 deaths caused by ovarian cancer (3.1 per 10000 person-years) in the intervention group and 100 deaths (2.6 per 10000 person-years) in the usual care group (mortality
RR,1.18; 95% CI, 0.82-1.71). Of 3285 women with false-positive results, 1080 underwent surgical follow-up; of whom, 163 women experienced at least 1 serious complication (15%). There were 2924 deaths due to other causes (excluding ovarian, colorectal, and lung cancer) (76.6 per 10000 person-years) in the intervention group and 2914 deaths (76.2 per 10000 person-years) in the usual care group (RR, 1.01;
95% CI, 0.96-1.06).
44
Effect of Screening on Ovarian Cancer Mortality
The Prostate, Lung, Colorectal and Ovarian (PLCO)
Cancer Screening Randomized Controlled Trial
Conclusions Among women in the general US population, simultaneous screening with CA-125 and transvaginal ultrasound compared with usual care did not reduce ovarian cancer mortality. Diagnostic evaluation following a false-positive screening test result was associated with complications.
Trial Registration clinicaltrials.gov Identifier: NCT00002540
JAMA. 2011;305(22):2295-2303
Published online June 4, 2011. doi:10.1001/jama.2011.766
45
Ovarian Cancer Risk
• Calculate the incidence of ovarian cancer in the screened group
• Incidence = (# new cases in screened)/(# screened) = 212/34253
= .00619
• Calculate the incidence of ovarian cancer in the usual care group
• Incidence = (#new cases in usual care)/(#women in usual care)
= 176/34304= .00513
46
Another Way to Frame the Dilemma
• Number needed to screen
• Definition - the number of people who need to be screened to prevent 1 death
• NNS = 1/absolute risk reduction (ARR)
• Absolute risk is the same as incidence…the number of new cases in a given group
• Absolute risk reduction is how much the risk decreases with a particular intervention
• In this study, it is the risk of ovarian cancer in the screening group minus the risk of ovarian cancer in the usual care group
47
Calculation Time!
• ARR = # new cases screened #new cases usual care total # screened total # usual care
= 212 176
34253 34304
= .00619 - .00513
= .
00106
48
NNS
• NNS
= 1/ARR
•
NNS = 1/.00106 =
943
•
Interpretation
• 943 women would need to be screened with TVUS and CA125 to prevent 1 ovarian cancer death
49
Case 3
• Carpenter CR., Keim SM., Milne WK., et al. (2010).
Thrombolytic therapy for acute ischemic stroke beyond three hours. The Journal of Emergency
Medicine, 40(1); 82-92.
50
Clinical Question
Does the intravenous systemic administration of tPA within 4.5 hours to select patients with acute ischemic stroke improve functional outcomes?
51
History of Present Problem
• NINDS 1995
• “alteplase…within 3 h of symptom onset significantly improved functionally independent outcomes at 3 months in highly select acute stroke patients.”
• Medical community response
• FDA approved in 1996
• AHA upgraded recommendation to Level 1 in 2000
• European Medicines Agency called for alteplase safety study in community setting and RCT of thrombolysis window beyond 3 hours
52
More Context
• Controversy in EM over Level I recommendation with only one study
• Other “real world” studies couldn’t reproduce the findings
• Ambiguous standard of care for those within 3 hours
• Re-analysis of NINDS data suggest benefit less substantial than originally believed
• Cochrane review overall benefit in spite of more deaths and intracranial hemorrhages
53
Bottom Line…
• We need to review the evidence for ourselves!
54
Efficacy and Safety of Tissue Plasminogen
Activator 3 to 4.5 Hours After Acute Ischemic
Stroke: A Meta-Analysis
Background and purpose — The ECASS-3 study demonstrated a benefit of treatment with intravenous tPA for acute stroke in the
3-4.5 hour time-window. Prior studies, however, have failed to demonstrate a significant benefit of tPA for patients treated beyond
3 hours. The purpose of this study was to produce reliable and precise estimates of the treatment effect of tPA by pooling data from all relevant studies.
Methods — A meta-analysis was undertaken to determine the efficacy of tPA in the 3-4.5 hour time window. The effect of tPA on favorable outcome and mortality was assessed .
55
Efficacy and Safety of Tissue Plasminogen
Activator 3 to 4.5 Hours After Acute Ischemic
Stroke: A Meta-Analysis
Results — The meta-analysis included data from patients treated in the 3-4.5 hour time-window in ECASS-1 (n=234), ECASS-2
(n=265), ECASS-3 (n=821) and ATLANTIS (n=302). tPA treatment was associated with an increased chance of favorable outcome (OR 1.31, 95% CI 1.10-1.56; p=0.002) and no significant difference in mortality (OR 1.04; 95% CI 0.75-1.43; p=0.83) compared to placebo treated patients .
Conclusions — Treatment with tPA in the 3-4.5 hour timewindow is beneficial. It results in an increased rate of favorable outcome without adversely affecting mortality
.
56
NINDS
• Population
• 37 hospitals, ischemic CVA, clear onset, < 3 hours
• Measurable sustained deficit NIHSS, no ICH
• Study design
• Randomized to placebo or alteplase
• Primary outcome
• 3mo functional outcome
• 4 instruments
• Those who died given worse possible score on all
57
NINDS
• Exclusion
• Extensive; see Table 1
• Main results
• 1 day outcome – better in those treated within 90 min
• 3 months
• 12% absolute increase in favorable outcomes (NNT = 8)
• No sig difference in mortality
• ICH
• More likely in tPA group (20/312) vs (2/312) placebo
58
ECASS I
• Population
• 75 hospitalized, 14 Euro countries, mod to high grade hemispheric stroke (SSS); < 6 hrs; no or min. infarct on
CT
• Study design
• Randomized to alteplase or placebo
• Primary outcome
• Barthel index and mRS at 3mo post-treatment
59
ECASS I
• Exclusion criteria
• See Table 2
• Main results
• In the ITT analysis, there was no difference in Barthel or mRS.
• mRS in TP analysis was significantly better in tPA group
• No significant difference in mortality
• Early ICH, fatal cerebral edema and early mortality were higher in treatment group
60
ECASS II
• Population
• 108 centers in 14 Euro countries, Australia & New Zealand;
• Mod to severe ischemic stroke; no or min. CT evidence; < 6 hrs.
• Study design
• Randomized to alteplase or placebo
• Primary outcome
• mRS at 90 days
61
ECASS II
• Exclusion criteria
• See Table 3
• Main results
• No sig difference in mRS (for score 0 or 1) at 3 months
• Including mRS = 2, tPA better with NNT = 12
• No difference in mortality at 3 months
• Parenchymal hemorrhage 4x higher in tPA
• Space occupying ICH 10x higher in tPA
• Hemorrhagic conversion of the strokes did not differ.
62
ATLANTIS
• Population
• 140 North American sites; ischemic stroke, measurable neuro deficit with ability to treat within 3-5 hours of onset
• Study design
• Randomized to tPA or placebo
• Primary outcome
• NIHSS score at 3 months
63
ATLANTIS
• Exclusion criteria
• See Table 4
• Main results
• No difference for NIHSS at 3 months.
• tPA significantly increased symptomatic and fatal
ICH…trend toward higher mortality at 3 months
64
ECASS III
• Population
• 130 sites, 19 Euro countries, clinically suspected acute ischemic stroke; 3-4.5h p sx onset
• Study Design
• Randomized to alteplase or placebo
• Primary Outcome
• 90 day mRS favorable outcome (score = 0 or 1)
65
ECASS III
• Exclusion
• See Table 5
• Main results
• mRS 0 or 1 in 52.4% of tPA vs. 45.2% of placebo
(NNT = 14)
• No significant difference in mortality rates
• Symptomatic ICH significantly more likely with tPA
(27% vs. 17.6%, NNH 47)
66
Meta Analysis
• Population
• Acute ischemic stroke treated with tPA in 3-4.5h window
• Study design
• Meta analysis of RCTs
• Primary outcome
• Global odds ratio (mRS 0-1, NIHSS 0-1, Barthel Index >=95)
• Good functional outcome at 90 days (mRS 0 or 1)
• 90 day mortality
6767676
7
Meta Analysis
• Exclusion criteria
• Observational studies and RCTs with n < 100
• Main results
• Global outcome
• Sig. better than placebo (OR 1.31, p = 0.002)
• mRS
• Sig better than placebo (OR 1.31, p = 0.008; NNT 15)
• No difference in 90 day mortality
68
69
Conclusion
• What is the conclusion of the article with regard to the use of thrombolytic therapy for ischemic stroke with symptom onset beyond three hours?
• “Treatment with tPA in the 3-4.5 hour time-window is beneficial. It results in an increased rate of favorable outcome without adversely affecting mortality”
• Is the conclusion supported by the NNTs for each set of data?
70
71
NNT
ECASS-1 13
ECASS-2 11
ECASS-3 14
ATLANTIS N/A
TOTAL 15
Is the Review Valid?
• Are there any concerns with the validity of this review?
• Size of meta analysis
• Heterogeneity of data
• Need CI to interpret NNT
72
Summary
• Questions of risk arise every day in clinical practice
• Clinicians need a reliable expression of risk that allows application of RCT results to individual patients and that makes sense is patients
• NNT, NNH and NNS are useful tools for this purpose
73
The NNT
• The NNT
• A group of physicians who have evaluated the evidence for a variety of therapies and diagnostics
• They provide a summary of the evidence and the bottom line benefits (NNTs) and harms (NNHs) scores
• Also includes a NNT tutorial
• www.theNNT.com
• The NNT quiz – let’s try it!
• http://www.thennt.com/the-nnt-intervention-quiz/
74
THE NNT.COM
• Their NNT rating system
75
Quiz Question 1
76
Quiz Question 1 Answer
77
Quiz Question 2
78
Quiz Question 2 Answer
79
Quiz Question 3
80
Quiz Question 3 Answer
81
Quiz Question 4
82
Quiz Question 4 Answer
83
Quiz Question 5
84
Quiz Question 5 Answer
85
Quiz Question 6
86
Quiz Question 6 Answer
87
Lingering Questions?
88