RENAL TUBULAR ACIDOSIS
advertisement

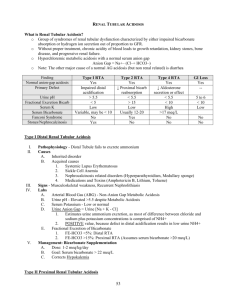
RENAL TUBULAR ACIDOSIS haiying liu department of nephrology the second hospital of shandong university • A 35 year old woman, a nursing home assistant, presents with chronic acidosis that is difficult to manage. Lab evaluation showed Na 143, K 2.8 Cl 118, HCO3 15 BUN 18, Cr 0.7. ABG reveals ph 7.38 Pco2 31, Pao2 100. U/A results were normal with urine ph of 5.0. Urine Na was 40 K 5 and Urine Cl 150.Which disorder best characterizes this pts syndrome. A. B. C. D. E. Diuretic abuse Laxative abuse Distal renal tubular acidosis Proximal renal tubular acidosis Type 4 renal tubular acidosis OUTLINE • Renal tubular acidosis (RTA) is applied to a group of transport defects in the reabsorption of bicarbonate (HCO3-), the excretion of hydrogen ion (H+), or both. • The RTA syndromes are characterized by a relatively normal GFR and a metabolic acidosis accompanied by hyperchloremia and a normal plasma anion gap. OBJECTIVES • • • • • Physiology of Renal acidification. Types of RTA and characteristics Lab diagnosis of RTA Approach to a patient with RTA Treatment Physiology of Renal Acidification • Kidneys excrete 50-100 meq/day of non carbonic acid generated daily. • This is achieved by H+ secretion at different levels in the nephron. • The daily acid load cannot be excreted as free H+ ions. • Secreted H+ ions are excreted by binding to either buffers, such as HPO42- and creatinine, or to NH3 to form NH4+. • The extracellular pH is the primary physiologic regulator of net acid excretion. 1. 2. Renal acid-base homeostasis may be broadly divided into 2 processes Proximal tubular absorption of HCO3(Proximal acidification) Distal Urinary acidification. Reabsorption of remaining HCO3- that escapes proximally. Excretion of fixed acids through buffering & Ammonia recycling and excretion of NH4+. Proximal tubule physiology • Proximal tubule contributes to renal acidification by H+ secretion into the tubular lumen through NHE3 transporter and by HCO3- reabsorption. • Approx. 85% of filtered HCO3- is absorbed by the proximal tubule. • The remaining 15 % of the filtered HCO3- is reabsorbed in the thick ascending limb and in the outer medullary collecting tubule. Proximal tubule physiology Multiple factors are of primary importance in normal bicarbonate reabsorption The sodium-hydrogen exchanger in the luminal membrane(NHE3). The Na-K-ATPase pump The enzyme carbonic anhydrase II & IV The electrogenic sodium-bicarbonate cotransporter(NBC-1). . Ammonia recycling • Ammonium synthesis and excretion is one of the most important ways kidneys eliminate nonvolatile acids. • Ammonium is produced via catabolism of glutamine in the proximal tubule cells. • Luminal NH4+ is partially reabsorbed in the thick ascending limb and the NH3 then recycled within the renal medulla Ammonia Recycling • The medullary interstitial NH3 reaches high concentrations that allow NH3 to diffuse into the tubular lumen in the medullary collecting tubule, where it is trapped as NH4+ by secreted H+. Distal Urinary Acidification • The thick ascending limb of Henle’s loop reabsorbs about 15% of the filtered HCO3load by a mechanism similar to that present in the proximal tubule, i.e., through Na+-H+ apical exchange(NHE3). H+ secretion • The collecting tubule (CT) is the major site of H+ secretion and is made up of the medullary collecting duct (MCT) and the cortical collecting duct (CCT). • Alpha and Beta-intercalated cells make up 40% of the lining while Principal cells and collecting tubule cells make up the remainder. • Alpha-Intercalated Cells are thought to be the main cells involved with H+ secretion in the CT. • This is accomplished by an apically placed H+K+-ATPase and H+-ATPase with a basolateral Cl-/HCO3- exchanger and the usual basolateral Na+ - K+ ATPase. • Beta-Intercalated Cells in contrast to the above have a luminal Cl-/HCO3- exchanger and a basolateral H+-ATPase. • They play a role in bicarbonate secretion into the lumen that is later reabsorbed by the CA IV rich luminal membrane of medullary collecting duct. • CCT H+ secretion is individually coupled to Na+ transport. Active Na+ reabsorption generates a negative lumen potential favoring secretion of H+ and K+ ions. • In contrast the MCT secretes H+ ions independently of Na+. • Medullary portion of the Collecting duct is the most important site of urinary acidification Principal cells Aldosterone and Renal acidification • Favors H+ and K+ secretion through enhanced sodium transport. • Recruits more amiloride sensitive sodium channels in the luminal membrane of the collecting tubule. • Enhances H+-ATPase activity in cortical and medullary collecting tubules. • Aldosterone also has an effect on NH4+ excretion by increasing NH3 synthesis Summary • H+ secretion, bicarbonate reabsorption and NH4+ production occur at the proximal tubule. Luminal CA IV is present in the luminal membrane at this site and in MCT. • NH4+reabsorption occurs at TAL of loop of Henle and helps in ammonia recycling that facilitates NH4+excretion at MCT. • H+ secretion occurs in the CCT either dependent or independent of Na availability and in the MCT as an independent process.. OBJECTIVES • • • • • Physiology of Renal Acidification. Types of RTA and characteristics Lab diagnosis of RTA Approach to a patient with RTA Treatment TYPES OF RTA Proximal RTA (type 2) • Isolated bicarbonate defect • Fanconi syndrome Distal RTA (type 1) • Classic type • Hyperkalemic distal RTA • Hyperkalemic RTA (Type 4) PROXIMAL RTA • Proximal RTA (pRTA) is a disorder leading to HCMA secondary to impaired proximal reabsorption of filtered bicarbonate. • Since the proximal tubule is responsible for the reabsorption of 85-90% of filtered HCO3- a defect at this site leads to delivery of large amounts of bicarbonate to the distal tubule. • This leads to bicarbonaturia, kaliuresis and sodium losses. • Thus patients will generally present with hypokalemia and a HCMA. . • Isolated defects in PCT function are rarely found. Most patients with a pRTA will have multiple defects in PCT function with subsequent Fanconi Syndrome. • The most common causes of Fanconi syndrome in adults are multiple myeloma and use of acetazolamide. • In children, cystinosis is the most common. • pRTA is a self limiting disorder and fall of serum HCO3_ below 12 meq/l is unusual, as the distal acidification mechanisms are intact.. • Urine ph become remains acidic(<5.5) mostly but becomes alkaline when bicarbonate losses are corrected. • FEHCO3 increases(>15%)with administration of alkali for correction of acidosis Cause of hyokalemia in Type 2 RTA Metabolic acidosis in and of itself decreases pRT Na+ reabsorption leading to increased distal tubule delivery of Na+ which promotes K+ secretion. The pRTA defect almost inevitably leads to salt wasting, volume depletion and secondary hyperaldosteronism. The rate of kaliuresis is proportional to distal bicarbonate delivery. Because of this alkali therapy tends to exaggerate the hypokalemia. • Patients with pRTA rarely develop nehrosclerosis or nephrolithiasis. This is thought to be secondary to high citrate excretion. • In children, the hypocalcemia as well as the HCMA will lead to growth retardation, rickets, osteomalacia and an abnormal vitamin D metabolism. In adults osteopenia is generally seen. DISTAL RTA • Distal RTA (dRTA) is a disorder leading to HCMA secondary to impaired distal H+ secretion. • It is characterized by inability to lower urine ph maximally(<5.5) under the stimulus of systemic acidemia. The serum HCO3- levels are very low <12 meq/l. • It is often associated with hypercalciuria, hypocitraturia, nephrocalcinosis, and osteomalacia. • The term incomplete distal RTA has been proposed to describe patients with nephrolithiasis but without metabolic acidosis. • Hypocitraturia is the usual underlying cause. • The most common causes in adults are autoimmune disorders, such as Sjögren's syndrome, and other conditions associated with chronic hyperglobulinemia. • In children, type 1 RTA is most often a primary, hereditary condition. Secretory defects causing Distal RTA Non secretory defects causing Distal RTA • Gradient defect: backleak of secretd H+ ions. Ex. Amphotericin B • Voltage dependent defect: impaired distal sodium transport ex. Obstructive uropathy, sickle cell disease, CAH, Lithium and amiloride etc. • This form of distal RTA is associated with hyperkalemia(Hyperkalemic distal RTA) • A high urinary pH (5.5) is found in the majority of patients with a secretory dRTA. • Excretion of ammonium is low as a result of less NH4+trapping. This leads to a positive urine anion gap. • Urine PCO2 does not increase normally after a bicarbonate load reflecting decreased distal hydrogen ion secretion. • Serum potassium is reduced in 50% of patients. This is thought to be from increased kaliuresis to offset decreased H+ and H-KATPase activity. Type 4 RTA (Hyperkalemic RTA) • This disorder is characterized by modest HCMA with normal AG and association with hyperkalemia. • This condition occurs primarily due to decreased urinary ammonium excretion. • Hypoaldosteronism is considered to be the most common etiology. Other causes include NSAIDS, ACE inhibitors, adrenal insufficiency etc. Mechanism of action • In contrast to hyperakalemic distal RTA, the ability to lower urine ph in response to systemic acidosis is maintained. • Nephrocalcinosis is absent in this disorder. OBJECTIVES • • • • • Physiology of Renal Acidification. Types of RTA and characteristics Lab diagnosis of RTA Approach to a patient with RTA Treatment Lab diagnosis of RTA • RTA should be suspected when metabolic acidosis is accompanied by hyperchloremia and a normal plasma anion gap (Na+ - [Cl- + HCO3-] = 8 to 16 mmol/L) in a patient without evidence of gastrointestinal HCO3- losses and who is not taking acetazolamide or ingesting exogenous acid. Functional evaluation of proximal bicarbonate absorption Fractional excretion of bicarbonate • Urine ph monitoring during IV administration of sodium bicarbonate. • FEHCO3 is increased in proximal RTA >15% and is low in other forms of RTA. Functional Evaluation of Distal Urinary Acidification and Potassium Secretion • • • • • • Urine ph Urine anion gap Urine osmolal gap Urine Pco2 TTKG Urinary citrate Urine ph • In humans, the minimum urine pH that can be achieved is 4.5 to 5.0. • Ideally urine ph should be measured in a fresh morning urine sample. • A low urine ph does not ensure normal distal acidification and vice versa. • The urine pH must always be evaluated in conjunction with the urinary NH4+ content to assess the distal acidification process adequately . • Urine sodium should be known and urine should not be infected. Urine Anion Gap • Urine AG = Urine (Na + K - Cl). • The urine AG has a negative value in most patients with a normal AG metabolic acidosis. • Patients with renal failure, type 1 (distal) renal tubular acidosis (RTA), or hypoaldosteronism (type 4 RTA) are unable to excrete ammonium normally. As a result, the urine AG will have a positive value. • There are, however, two settings in which the urine AG cannot be used. • When the patient is volume depleted with a urine sodium concentration below 25 meq/L. • When there is increased excretion of unmeasured anions Urine osmolal gap • When the urine AG is positive and it is unclear whether increased excretion of unmeasured anions is responsible, the urine ammonium concentration can be estimated from calculation of the urine osmolal gap. • UOG=Uosm - 2 x ([Na + K]) + [urea nitrogen]/2.8 + [glucose]/18. • UOG of >100 represents intact NH4 secretion. Urine Pco2 • Measure of distal acid secretion. • In pRTA, unabsorbed HCO3 reacts with secreted H+ ions to form H2CO3 that dissociate slowly to form CO2 in MCT. • Urine-to-blood pCO2 is <20 in pRTA. • Urine-to-blood pCO2 is >20 in distal RTA reflecting impaired ammonium secretion. TTKG • TTKG is a concentration gradient between the tubular fluid at the end of the cortical collecting tubule and the plasma. • TTKG = [Urine K ÷ (Urine osmolality / Plasma osmolality)] ÷ Plasma K. • Normal value is 8 and above. • Value <7 in a hyperkalemic patient indicates hypoaldosteronism. • This formula is relatively accurate as long as the urine osmolality exceeds that of the plasma urine sodium concentration is above 25 meq/L Urine citrate • The proximal tubule reabsorbs most (70-90%) of the filtered citrate. • Acid-base status plays the most significant role in citrate excretion. • Alkalosis enhances citrate excretion, while acidosis decreases it. • Citrate excretion is impaired by acidosis, hypokalemia,high–animal protein diet and UTI. OBJECTIVES • • • • • Physiology of Renal acidification. Types of RTA and characteristics Lab diagnosis of RTA Approach to a patient with RTA Treatment OBJECTIVES • • • • • Physiology of Renal acidification. Types of RTA and characteristics Lab diagnosis of RTA Approach to a patient with RTA Treatment Treatment • Proximal RTA • A mixture of Na+ and K+ salts, preferably citrate, is preferable. • 10 to 15 meq of alkali/kg may be required per day to stay ahead of urinary losses. • Thiazide diuretic may be beneficial if large doses of alkali are ineffective or not well tolerated. Distal RTA • Bicarbonate wasting is negligible in adults who can generally be treated with 1 to 2 meq/kg of sodium citrate (Bicitra) or bicarbonate. • Potassium citrate, alone or with sodium citrate (Polycitra), is indicated for persistent hypokalemia or for calcium stone disease. • For patients with hyperkalemic distal RTA, highsodium, low-potassium diet plus a thiazide or loop diuretic if necessary. Hyperkalemic RTA • Treatment and prognosis depends on the underlying cause. • Potassium-retaining drugs should always be withdrawn.. • Fludrocortisone therapy may also be useful in hyporeninemic hypoaldosteronism, preferably in combination with a loop diuretic such as furosemide to reduce the risk of extracellular fluid volume expansion.