Molecules & Ions
advertisement

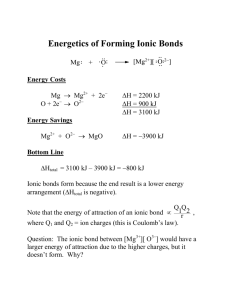
Molecules and Ions AP Chemistry, Unit 2, Presentation 1 Image courtesy of www.lab-initio.com Molecules Two or more atoms of the same or different elements, covalently bonded together. Molecules are discrete structures, and their formulas represent each atom present in the molecule. Pentane, C5H12 Covalent Network Substances Covalent network substances have covalently bonded atoms, but do not have discrete formulas. Why Not?? Graphene – carbon allotrope Ions Cation: A positive ion Mg2+, NH4+ Anion: A negative ion Cl-, SO42Ionic Bonding: Force of attraction between oppositely charged ions. Ionic compounds form crystals, so their formulas are written empirically (lowest whole number ratio of ions). Predicting Ionic Charges Group 1: Lose 1 electron to form 1+ ions H+ Li+ Na+ K+ Rb+ Cs+ Predicting Ionic Charges Group 2: Loses 2 electrons to form 2+ ions Be2+ Mg2+ Ca2+ Sr2+ Ba2+ Predicting Ionic Charges B3+ Al3+ Ga3+ Group 13: Loses 3 electrons to form 3+ ions Predicting Ionic Charges C22- C4- Caution! and are both called carbide Group 14: Loses 4 electrons or gains 4 electrons Predicting Ionic Charges N3- Nitride P3- Phosphide As3- Arsenide Group 15: Gains 3 electrons to form 3- ions Predicting Ionic Charges O2- Oxide S2- Sulfide Se2- Selenide Group 16: Gains 2 electrons to form 2- ions Predicting Ionic Charges F1- Fluoride Cl1- Chloride Br1- Bromide I1- Iodide Group 17: Gains 1 electron to form 1- ions Predicting Ionic Charges Group 18: Stable Noble gases do not form ions! Predicting Ionic Charges Groups 3 - 12: Many transition metals have more than one possible oxidation state. Iron(II) = Fe2+ Iron(III) = Fe3+ Predicting Ionic Charges Groups 3 - 12: Some transition metals have only one possible oxidation state. Zinc = Zn2+ Silver = Ag+ Writing Ionic Compound Formulas Example: Barium nitrate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Ba2+ ( NO3- ) 2 Not balanced Writing Ionic Compound Formulas Example: Ammonium sulfate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. ( NH4 +) 2 SO42- Not balanced Writing Ionic Compound Formulas Example: Iron(III) chloride 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Fe3+ Cl- 3 Not balanced Writing Ionic Compound Formulas Example: Aluminum sulfide 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. 3+ Al 2 2S Not balanced 3 Writing Ionic Compound Formulas Example: Magnesium carbonate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 2+ Mg CO32- They are balanced Writing Ionic Compound Formulas Example: Zinc hydroxide 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Zn2+ ( OH- ) 2 Not balanced Writing Ionic Compound Formulas Example: Aluminum phosphate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. Al3+ PO43They ARE balanced Naming Ionic Compounds Cation first, then anion Monatomic cation = name of the element Ca2+ = calcium ion Monatomic anion = root + -ide Cl- = chloride CaCl2 = calcium chloride Naming Ionic Compounds Metals with multiple oxidation states some metal forms more than one cation use Roman numeral in name PbCl2 Pb2+ is cation PbCl2 = lead(II) chloride Binary Molecular Compounds Compounds between two nonmetals First element in the formula is named first. Keeps its element name Gets a prefix if there is a subscript on it Second element is named second Use the root of the element name plus the -ide suffix Always use a prefix on the second element 1 = mon(o) 2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deka List of Prefixes Naming Binary Compounds P2O5 = diphosphorus pentoxide CO2 = carbon dioxide CO = carbon monoxide N2O = dinitrogen monoxide Practice – Write the Formula Compound Name Compound Formula Carbon dioxide Carbon monoxide Diphosphorus pentoxide Dinitrogen monoxide Silicon dioxide Carbon tetrabromide Sulfur dioxide Phosphorus pentabromide Iodine trichloride Nitrogen triiodide Dinitrogen trioxide Check next slide for answers Answers – Write the Formula Compound Name Compound Formula Carbon dioxide CO2 Carbon monoxide CO Diphosphorus pentoxide P2O5 Dinitrogen monoxide N2O Silicon dioxide SiO2 Carbon tetrabromide CBr4 Sulfur dioxide SO2 Phosphorus pentabromide PBr5 Iodine trichloride ICl3 Nitrogen triiodide NI3 Dinitrogen trioxide N2O3 Practice – Name the Compounds Compound Formula Compound Name N2O4 SO3 NO NO2 As2O5 PCl3 CCl4 H2O SeF6 Check next slide for answers Answers – Name the Compounds Compound Formula Compound Name N2O4 dinitrogen tetroxide SO3 sulfur trioxide NO nitrogen monoxide NO2 nitrogen dioxide As2O5 diarsenic pentoxide PCl3 phosphorus trichloride CCl4 carbon tetrachloride H2O dinitrogen monoxide SeF6 selenium hexafluoride