Atomic
advertisement

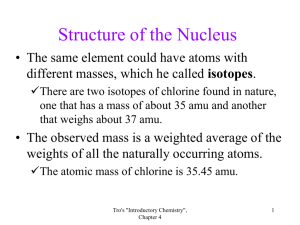
Determines the element Number of protons CANNOT change for an element Common charge notation is +1 Mass (g) = 1.673 x 10-24 Mass (amu)= 1.0073 = 1 Common charge notation is 0. No charge! Mass (g) 1.675 x 10-24 (same mass as proton) Mass (amu)= 1.0082 = 1 Number of neutrons CAN change for an element ISOTOPES Stabilizes nucleus by nuclear force Common Charge notation is -1 Mass (g) of e- = 9.109 x 10-28 Mass (amu)= .0006 = 0 Number of electrons CAN change for an element ISOTOPES Responsible for chemical reactivity, forms bonds Proton Neutron Electron Nucleus Nucleus Outside of nucleus Common charge + 0 _ Mass, g 1.673x10-24 1.675 x 10-24 9.109 x 10-24 Atomic mass unit, amu 1.0073 1 1.0082 1 .0006 0 Location Which subatomic particle can NOT change for an element and gives its identity? Atomic Number: The number of protons in each atom of that element. The atomic number is located above the element symbol. Unique to EACH element, never changes!! Isotopes: Are atoms of the same element that have different masses. An Isotope of an element has different number of neutrons. Example of isotopes: Mass Number: The total number of protons and neutrons that make up the nucleus of an isotope. Common isotopes of carbon Carbon-12 Carbon-13 *12, 13, and 14 are mass numbers* Carbon-14 Element- Mass number Ions :are when the electron number and proton number are different leading to a net charge. NOTE: YOU WILL ALWAYS ASSUME ATOMS ARE NEUTRAL (protons = electrons) UNLESS TOLD OTHERWISE Atomic #= Proton #= Electron # (because we assume atoms are neutral) Mass #= Atomic # + Neutron # Number of protons= Atomic number Number of electrons = Number of protons (in a neutral atom) Number of neutrons = Mass number– Atomic number Symbol Atomic # Na 11 15 Mass # 31 # of protons # of electrons # of neutrons Average atomic mass is the weighted average of the atomic masses of the naturally occurring isotopes of an element This number appears below the element symbol on the periodic table.