Concepts in Biochemistry 3/e
advertisement

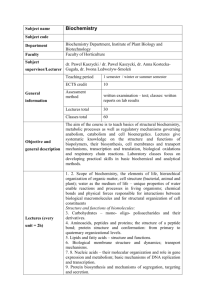
Zatilfarihiah Rasdi Biochemistry I BCM201 Chapter 1: An Introduction to Biochemistry OBJECTIVES Upon completion of this course, students should be able to: Define the basic constituents of macromolecules (carbohydrates, a. acids, lipids, nucleic acids) and enzymes Describe the classification, structure and functions of macromolecules and enzymes Explain and identify the basic energy generated processes Demonstrate the ability to conduct basic biochemical laboratory test Verbally and in writing, discuss and report to peers the scientific investigations and data interpretation CREDIT HOURS: 3 Assessment •Tests - 30% •Lab reports/assign.- 10% •Final exam. - 50% Lecture (DFT): 2hrs/week Friday : 10.10 am – 12.00 pm Venue: BK5 Practical: 3hrs/week Friday: 3.10 – 6.00 pm Venue: M. Biokimia LESSON PLAN Week Contents 1 1.0 Introduction to biochemistry 2.0 Carbohydrates 2 2.0 Carbohydrates 3 3.0 Lipid 4 4.0 Amino acid 5 4.0 Amino acid 5.0 Protein 6 5.0 Protein 7 5.0 Protein 8 6.0 Enzymes 9 6.0 Enzymes 10 Mid term break (Eid break) 11 7.0 Nucleic acid 12 7.0 Nucleic acid 13 8.0 Overview of metabolism 14 8.0 Overview of metabolism 15 Study week 16 Final examination LAB CLASS Reference Colorimetric tests for Campbell, M.K., and Farrell, S.O. carbohydrates (2009) Biochemistry, 6th ed. Chromatography Thompson Brooks/Cole Saponification value Boyer, R. (2005) Acid value Concepts in Biochemistry, 3rd ed. Qualitative tests for Wiley amino acid Tests for proteins Additional text The pH meter Voet, D.J, Voet, G.V, and Pratt, C.W Paper chromatography (2008) Principle of Biochemistry, 3rd ed. Wiley INTRODUCTION TO BIOCHEMISTRY BIOCHEMISTRY? The study of life at the molecular level It emerged as a distinct discipline around the beginning of the 20th century when scientists combined chemistry, biology and physiology to investigate the chemistry of living system Goal To describe life’s processes using the language of molecules – applying the principles and methods of chemistry to determine molecular structure from which it is often possible to explain biological function 1. 2. 3. Structural and functional biochemistry focuses on discovering chemical structures & 3-D arangements of biomolecules Informational biochemistry defines language(s) for storing biological data & trnasmitting it in cells and organisms Bioenergetics the flow of energy in living organisms and how it transferred from one process to another – study of metabolism WHY BIOCHEMISTRY? lead us to fundamental understanding of life, how organisms store & transfer information, how food digested, how brain cell store information…. • Understand the important issues in medicine, health & nutrition – can search cures for HIV, diabetes, recombinant DNA help in find new mutation and new plant • Advance biotechnology industries - The application of biological materials to technical useful operation e.g. enzymes in pharmaceutical industry to synthesise complex drugs • 1. BIOMOLECULES Field of biochemistry draws many disciplines – allows us to answer questions related to molecular nature of life Organic chemistry: the study of the compounds of carbon A biomolecule is a molecule that naturally occurs in living organisms. - biomolecules consists primarily of carbon and hydrogen, along with nitrogen, oxygen, phosphorus and sulfur. - important classes of biomolecules: proteins, carbohydrates, lipids, nucleic acids. Living cells include very large molecules, such as proteins, nucleic acids, polysaccharides and lipids. - biomolecules are polymers (Greek: poly + meros, many + parts) - are derived from monomers (Greek: mono + meros, single + part) a.acids (proteins), nucleotides (nucleic acids), monosaccharides (polysaccharides), glycerol and fatty acids (lipids) Fig. 1-5: Two natural organometallic compounds a) heme, containing a porphyrin ring and iron; b) chlorophyll, containing a porphyrin ring and magnesium BIOMOLECULES Enzymes: a class of proteins that are biocatalysts - the catalytic effectiveness of an enzyme depends on its amino acid sequence Genetic code: the relationship between the nucleotide sequence in nucleic acids and the amino acid sequence in proteins – BCM 301 Biomolecules Functional group: an atom/group of atoms that shows characteristi c, physical and chemical properties Homopolymers Heteropolymers Fig. 1-6:Types of natural polymers Viruses: consist of a single DNA or RNA molecule wrapped in a protein package Not considered a life-form Deemed parasites – unable to carry out metabolism or reproduction without the assistance of host cell Are the caused of many plants and animals maladies and has resulted in much human suffering However, an enormous study of biochemistry has been learned from studies of their actions 2. BIOLOGICAL INFORMATION Biochemists and molecular biologists have been interested in learning how biological information is transferred from one generation to another. DNA, RNA, proteins and even some carbohydrates are information-rich molecules that carry instructions for cellular processes. Biochemists work with the molecules, cell components and cells in a wide range of sizes. The Watson and Crick double helix model for DNA showing the stacking of nucleotide bases on the same strand and the hydrogen bonds between complimentary nucleotide bases on opposite strands. Biochemistry is an experimental science. Before doing experiment, the desired object of study must be separated from its natural surroundings – one tool useful for isolating cells, organelles and biomolecules is centrifuge. Scientists take advantage of the fact that biochemical entities have different physical characteristics; weight, sizes, densities, and shapes. • New biochemical research is showing that obesity not just a consequence of overeating but is often linked to malfunctions in the hormone-controlled systems that regulated energy consumption and body weight. ASSIGNMENT 1 (in group of 4/5) 1. Biological information flow from DNA RNA Protein. Write about the whole process from replication to translation to protein in 100-200 words. 2. Discuss the uses of biochemistry in environmental science, gene engineering & cloning and clinical chemistry.