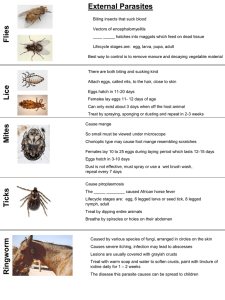
MEDICAL PROTOZOOLOGY
advertisement

TOPIC: INTRODUCTION TO MEDICAL PARASITOLOGY Parasitology is the study of parasites and as such that does not include bacterial, fungal or viral parasites. Human parasites are separated into intestinal and blood borne parasites. For a parasite to be defined as intestinal it must have an intestinal life cycle stage, though it may have life-cycle stages in the heart, blood vessels, and lungs in the humans, other animals or the environment. The association between two organisms may be one of the following: MUTUALISM: mutual benefit is derived from the association. SYMBIOSIS: mutual benefit, but the two organisms cannot live independently. COMMENSALISM: one partner benefits (commensal) while the other (host) is unaffected. It may be called a non-pathogenic parasite. When an animal lives on another organism from which it receives food and shelter without any compensation to it, and then this association is called parasitism. The animal, which enjoys advantages, is the parasite. All animals have parasites; hence there are more parasites than free-living animals. The habitat occupied by a parasite is very different from the environment of its free-living ancestors, hence it has either to adapt itself to this new habitat or perish. PARASITISM: one organism (parasite) lives at the expense of the other (host). The latter usually suffers from the association with pathogenic parasite). Parasitism is the form of mutual relations between organisms of various kinds, from which one (parasite) uses another (host) as environment for living, and from which it obtains food causing him damage (disease). PARASITOLOGY is a complex biological science studying the phenomena of parasitism. There are mutual relations between the parasite and host, their dependence on the factors of external environment, and also diseases, caused by the parasites, and methods of fighting with them in man, animals and plants. MEDICAL PARASITOLOGY consists of 3 parts: medical protozoology, medical helminthology and medical entomology. CLASSIFICATION OF PARASITES Each parasite belongs to a phylum, class, order, family, genus and species; the scientific designation of a parasite is binomial, a generic name (genus) and a specific name (species). The parasites of humans in the kingdom protozoa are now classified under 3 phyla: Sarcomastigophora (containing the amoebae and flagellates); Apicomplexa (containing the sporozoan); and Ciliophora (containing the ciliates). The important human parasites are found within these great groups. 1. Class LOBOZEA (Sarcodina) is typically amoeboid and in represented in humans by class of Entamoeba, Endolimax, lodamoeba, Naegleria, and Acanthamoeba. 2. Class ZOOMASTIGOPHORA, the flagellates, have one or more whip-like flagella and, in some cases, an undulating membrane (e.g., trypanosomes). These include intestinal and genitourinary flagellates (Giardia, Trichomonas, Dientamoeba, Chilomastix) and blood tissue flagellates (Trypanosoma, Leishmania). 3. Class SPOROZOA undergoes a complex life cycle with alternating sexual and asexual reproductive phases, usually involving two different hosts (e.g., arthropod and vertebrate, as in the blood forms). The subclass Coccidia contains the human parasites Isospora, Toxoplasma, and others. One of these, Cryptosporidium, has been implicated as a cause of intractable diarrhea among the immunosuppressed. Among the Haemosporina (blood sporozoan) are the malaria parasite (Plasmodium species) and the subclass Piroplasmia, which includes Babesia species. Pneumocystis has recently been shown to be a member of the Fungi rather than the Protozoa. It is another opportunistic parasite of immunosuppressed individuals. 4. Class LITOSTOMATEA is a complex protozoan bearing cilia distributed in rows or patches, with two kinds of nuclei in each individual. Balantidium coli, a giant intestinal ciliate of humans and pigs, is the only human parasite representative of this group. THE PARASITIC WORMS, or helminths, of a human being, belong to two phyla: 1. PLATYHELMINTHS (flatworms) lack a true body cavity (celom) and are characteristically flat in dorsoventral section. Medically important species belong to the classes Cestoda (tapeworms) and Trematoda (flukes). The tapeworms of humans are band-like and segmented; the flukes are typically leaf-shaped, and the schistosomes are narrow and elongate. The important tissue and intestinal cestodes of humans belong to the genera Diphyllobothrium, Spirometra, Taenia, Echinococcus, Hymenolepis, and Dipylidium. Medically important trematode genera include Schistosoma, Paragonimus, Clonorchis, Opistorchis, Heterophyes, Metagonimus, Fusciolopsis, and Fasciola. 2. NEMATHELMINTHS (worm-like, separate-sexed, insegmented roundworms) include many parasitic species that infect humans. PHYLUM ARTHROPODA includes 3 classes of medical importance: 1. CLASS CRUSTACEA: Cyclops, etc. 2. CLASS ARACHNIDA (Octapoda): scorpions, ticks and mites. 3. CLASS INSECTA (Hexapoda): mosquitoes, flies, bugs, lice and fleas. TYPES OF PARASITES ENDOPARASITE: lives within the body of the host (infection). ECTOPARASITE: lives on the outside of the body of the host (infestation). OBLIGATE PARASITE: completely dependent upon its host and cannot lead a free life. FACULTATIVE PARASITE: capable of leading both a free-living and a parasitic existence. INCIDENTAL PARASITE: can establish itself in a host in which it does not ordinarily live. ACCIDENTAL PARASITE: a free-living organism that may live as a parasite in a host. PERMANENT PARASITE: remains all or most of its life in or on its host. TEMPORARY PARASITE (occasional or intermittent): free-living but seeks its host from time to time for food. PERIODIC PARASITE (transitory): passes a definite part of its life cycle as a parasite. SPECIFIC PARASITE: occurs in one particular host. PSEUDOPARASITE: an artifact mistaken for a parasite. COPROZOIC or SPURIOUS PARASITE: a free-living or a non-human parasite passing through the alimentary canal without infecting man or contaminating his stools after being passed. OPPORTUNISTIC PARASITE: occurs in patients with impaired host defense (immunodeficient or immunocompromised host). Such parasite does not ordinarily cause disease in healthy immunocompetent) individuals. TYPES OF HOSTS 1. FINAL HOST or DEFINITIVE HOST: harbours the adult or sexually mature parasite. 2. RESERVOIR HOST: an animal that harbours the same species of parasites as man and constitute a source of infection to him. Usually these animals are not affected by infection. The reservoir host serves as a potential source of human reinfection and as means of sustaining the parasite when it is not infecting man. Diseases of animals that are transmissible to man are called zoonotic diseases. 3. INTERMEDIATE HOST: harbours the immature or asexual stages of the parasite. 4. VECTOR: an arthropod that carries a parasite to its host. Source and mode of parasitic infection or infestation 1. Food and drink: Ingestion of raw or undercooked food or drinking water or other beverages containing the infective stage of the parasite. 2. Soil, dust and water (canals and streams) a) Ingestion of food or drink contaminated with soil or dust containing the infective stage of the parasite. b) Inhalation of dust. c) Direct contact with soil (handling, walking barefooted); the infective stage penetrates the skin. d) Using water streams (washing, swimming, wading, irrigation, etc.); the infective stage penetrates the skin or mucous membrane. 3. Vector a) Bite of vector inoculating the infective stage. b) Feces of vector containing the infective stage contaminate the skin (intact or wounded). c) Ingestion of vectors containing the infective stage. d) Direct penetration of an arthropod into the skin. 4. Direct contact: a) Skin contact. b) Sexual contact. c) Autoinfection or direct infection. 5. Congenital infection. Effect of the parasite on the host (Pathogenicity) The effect depends on the number, size and shape of the parasite; its activity (movement and migration); site (habitat); specific toxin and host reaction. The effect may be due to: 1. The parasite abstracting nourishment from the host. 2. Mechanical effect leading to tissue destruction as a result of trauma, pressure, compression or obstruction; feeding on tissues or irritation of tissues leading to inflammatory or neoplastic reactions. 3. Toxic effects from toxins secreted or waste products excreted by the parasite, leading to poisoning or allergic reactions. 4. Secondary infection with other organisms as bacteria. The host reaction to the invading parasite may be: a) Generalized in the form of fever, anaemia, leucocytosis, leucopaenia, eosinophilia, allergic reactions, weakness, etc. b) Localized according to the tissue or organ affected, e.g. gastrointestinal disturbances (colic, dyspepsia, diarrhea, and dysentery), itching, ulceration, hepatomegaly, splenomegaly, etc. TOPIC: PROTOZOA MEDICAL PROTOZOOLOGY Protozoa are single celled organisms. There are four classes of Protozoa commonly found in concentrated faecal samples. These are differentiated by the method of motility. Protozoa include Entamoeba, Giardia, Trichomonas, Cryptosporidium, Isospora, Pneumocystis and Balantidium. There are two diagnostic life-cycle stages commonly seen in parasites – the cyst and the adult trophozoite stage. The trophozoite stage is analyzed directly on a slide without concentration. Cysts require concentration. The key diagnostic factor is that Protozoan cysts are typically 5-30 microns in diameter, and as such that are smaller than most Helminths eggs. Due to the size they are particularly difficult to see under the microscope if the sample clarity is bad. The medically important Helminths are nematodes (roundworms), cestodes (tapeworms) and trematodes (flukes). Genera include: Fasciola, Schistosoma, Ascaris, Hookworm, Trichuris, Taenia and Enterobius. The normal stage for examination is the egg stage, although larvae may develop in some organisms (Strongyloides). The diameter of the eggs ranges from 30-150 microns. The other major groups of parasites are known as blood-borne parasites where they are transmitted by an arthropod vector. Far more important arthropods for transmitting parasitic infections are the mosquitoes. Mosquitoes are known to carry malaria and filarial nematodes. Different types of biting flies transmit African trypanosomiasis, leishmaniasis and several kinds of filariasis. Most protozoan and helminthic infections that are transmitted by arthropods can readily be diagnosed, on clinical basis alone, but are usually identified by fairly simple techniques designed to reveal the presence of the causative parasite by microscopy. Sophisticated techniques are also being employed including highly sensitive and specific simple monoclonal antibody tests, DNA probes and PCR primers. Protozoa exhibit a wide variety of morphologies. There is no one shape or morphology, which would include a majority of protozoa. Shapes range from the amorphous and ever-changing forms of amoeba to relatively rigid forms dictated partially by highly ordered cytoskeletons or secreted walls or shells. Many protozoan species exhibit complex life cycles with multiple stages. Sometimes the different life cycle stages are so dissimilar that they have been mistaken for completely different species. The vast majority of protozoa are microscopic. However, they do exhibit an incredibly large range of sizes. Extant species range in size from < 1 μm (10-6 meter) to several mm. Fossilized Forminiferida of several cm have been identified. Most of the organisms discussed in Parazitology will be 3 – 50 μm. This small size necessitates the use of a microscope to detect protozoa. An electron microscope is needed for detailed morphological studies. Protozoa are found virtually everywhere. As a group, the protozoa are extremely adaptable. Individual species, though, may have very specific-niches. Like all other organisms, protozoa must be able to acquire and metabolize nutrients from their environment. Many protozoa simply absorb fluids (i.e., osmotrophy) from their media, while some are scavengers that ingest solid material (i.e., phagotrophy). Predatory protozoa either actively hunt down or passively ambush other organisms (typically bacteria or other protozoa). Some protozoa are photosynthetic and can capture the energy of the sun and convert it to usable chemical energy (i.e., autotrophic or phototrophic). Many protozoa are not restricted to a single feeding mechanism and can utilize combinations mentioned above (i.e., heterotrophic, mixotrophic). Protozoa are conveniently divided into free-living and symbiotic with a few that are facultative symbionts. Generally free-living organisms are found in the soil or aqueous environments, whereas symbionts live in close association with another organism. Symbiosis implies a physiological dependency on another organism for its nutrition. Different forms of symbiosis can be distinguished on the nature of the association between the dissimilar organisms: commensalism: denotes an interaction that is beneficial to one organism but has no affect on the other organism. For example, many protozoa live in the alimentary canal of another organism without doing any harm. These commensals are often scavengers or predators that exploit the abundance of nutrients or bacterial fauna provided by the host organism; mutualism: is a special form of commensalism in which both organisms derive some benefit and become dependent on each other. The classic example of mutualism is the protozoan Trichonympha found in the gut of termites. Trichonympha, with the assistance of symbiotic bacteria, digests the wood particles (i.e., cellulose) ingested by the termite; parasitism: denotes a relationship in which one organism (the parasite) benefits at the expense of the other organism (the host). Generally this host expense implies that the parasite takes in macromolecules from the host and releases others into the host. In some instances the parasitism will be overtly harmful to the host and referred to as being pathogenic. These pathogenic protozoa will be the primary focus of this course. The earliest observations of protozoa noted their motility. However, motility is not a universal feature of protozoa and different protozoa utilize different mechanisms for their movement. In fact, protozoa were initially classified based in part on their motility. Cilia and flagella are subcellular structures, which propel protozoa through a fluid medium. Flagella are long whip-like structures, which propel the organism as a result of wave-like beat, which is propagated through their length. Flagellated protozoa typically have one or a few flagella per organism. In contrast, ciliated protozoa are usually covered with rows of numerous cilia. Cilia and flagella can also assist in the procurement of food, reproduction and other functions. In contrast to the swimming exhibited by flagellates and ciliates, amoeba are protozoa that crawl along a solid substratum in a fashion known as “amoeboid movement”. The amoeba projects out a pseudopod, or false foot, and then pulls the rest of the body forward. The most common form of reproduction in protozoa is asexual binary fission. In other words, a single organism will divide into two equal organisms. A slight modification of this binary fission, called budding, is when one of the newly formed cells is smaller than the other. Typically the larger cell is called the mother and the smaller is the daughter. Some protozoa will form an intracellular bud and essentially give birth. Another variation of binary fission is a multiple fission or segmentation. In this situation, several rounds of nuclear replication occur without cytokinesis. This multinucleated cell will then form multiple progeny simultaneously. In summary, protozoa are unicellular eukaryotic microorganisms. However, the amount of diversity concerning morphology, size and life styles exhibited by protozoa makes it difficult to develop a more precise definition. However, protozoa do exhibit many of the features found in other eukaryotes. PROTOZOAN TAXONOMY Taxonomy is concerned with the classification and denomination of organisms. In addition to nomenclature, taxonomy also places organisms into groups, sharing common features, and presumably evolutionary relationships. However, taxonomic criteria are often arbitrary, and taxonomy is always changing to reflect new discoveries and interpretations. Protozoa are usually classified within the Kingdom Protista (Haeckel, 1866), which includes other unicellular eukaryotes. Historically protozoa were divided into four major groups: the amoebae, the flagellates, the sporozoa and the ciliates. The distinguishing features between the groups were based on motility (i.e., amoeboid, flagella, cilia). The sporozoa were a heterogeneous group that produced spores during one stage of their life cycles and exhibited “gliding” motility. In addition, some members within these two groups share some common features and therefore the amoebae and flagellates can be grouped together (e.g., sarcomastigophora). So, the parasites of humans in the subkingdom PROTOZOA are now classified under 3 phyla: SARCOMASTIGOPHORA (containing the amoebae and flagellates); APICOMPLEXA (containing the sporozoans); and CILIOPHORA (containing the ciliates). The important human parasites are found within these great assemblages. 1. Class LOBOZEA (Sarcodina) is typically amoeboid and is represented in humans by species of Entamoeba, Endolimax, Iodamoeba, Naegleria, and Acanthamoeha. 2. Class ZOOMASTIGOPHORA, the flagellates, have one or more whip-like flagella and, in some cases, an undulating membrane (e.g., trypanosomes). These include intestinal and genitourinary flagellates (Giardia, Trichomonas, Dientumoeba, Chilomastix), and blood and tissue flagellates (Trypanosoma, Leishmania). 3. Class SPOROZOA undergoes a complex life cycle with alternating sexual and asexual reproductive phases, usually involving two different hosts (e.g., arthropod and vertebrate, as in the blood forms). The subclass Coccidia contains the human parasites Isospora, Toxoplasma, and others. Among the Haemosporina (blood sporozoans) are the malaria parasites (Plasmodium species). 4. Class CILIOPHORA is a complex protozoon bearing cilia distributed in rows or patches, with two kinds of nuclei in each individual. Balantidium coli, a giant intestinal ciliate of humans and pigs, is the only human parasite representative of this group. GENERAL MORPHOLOGY OF PROTOZOA 1. Protozoa are unicellular organisms (single cell) sometimes called noncellular or acellular, being not divided into cells. 2. Each protozoon performs all vital functions. 3. The protozoon is made of a mass of protoplasm differentiated into cytoplasm and nucleoplasm. 4. The cytoplasm consists of outer thin hyaline ectoplasm and inner voluminous granular endoplasm. 5. The ectoplasm perfoms the following: protection, locomotion, and ingestion of food, excretion and respiration. 6. The endoplasm is concerned with metabolism. It encloses: a) food vacuoles: containing food during digestion, b) volutin granules: stored food in the form of carbohydrate (glycogen vacuoles) or protein (chromatoid bodies), c) excretory vacuoles: collect waste products and discharge them to the exterior by bursting through the ectoplasm or by an anal opening (cytopyge), d) the nucleus. 7. The nucleus is important in reproduction and maintaining life. It is made of nuclear membrane, nuclear sap, and chromatin. In the vesicular nucleus chromatin is concentrated in a mass (the karyosome) or distributed between the karyosome and the inner surface of the nuclear membrane (peripheral chromatin). In the massive nucleus chromatin is distributed diffusely. GENERAL BIOLOGY OF PROTOZOA 1. Nutrition is perfomed either by: absorption of liquid food through the body surface, or ingestion of solid particles by means of pseudopodia or through the cytostome. 2. Excretion is perfomed either by: diffusion through the body surface, or excretory vacuoles. 3. Secretion: digestive enzymes, toxins, and material for cyst wall, enzyme to liquefy tissues. 4. Respiration is either: aerobic or anaerobic. 5. Locomotion can be carried by: a) Pseudopodia (amoeboid movement); b) Flagella: whip-like filaments arise from the kinetoplast+ blepharoplast + parabasal body); c) Cilia: like flagella but smaller and more numerous covering most of the body. 6. Cyst formation: encystment of some protozoa is essential for survival outside the body of the host and during transmission from host to host. 7. Reproduction may be asexual or sexual. a) Asexual reproduction (simple fission): division of the nucleus by amitosis (simple division) or mitosis (differentiation of chromatin into chromosomes); division of the cytoplasm by simple fission into two (binary fission) or more (multiple fission). b) Sexual reproduction: the formation of 2 cells (male and female gametes) by reduction division, and their union (or syngamy) resulting in the formation of a zygote. The Amoebae Amoebae are characterized by possessing clear protoplasm, which forms pseudopodia. These pseudopodia are the means by which these organisms move and engulf bacteria and red blood cells for feeding purposes. The most common amoebae seen in the intestinal tract are Entamoeba histolytica, Entamoeba coli, Entamoeba hartmanni, Endolimax nana and Iodamoeba. All but Entamoeba histolytica are thought to be non-pathogenic. The cysts can be identified in an ethyl acetate concentrate by addition of iodine to reveal the characteristic inclusions and also by measuring the cyst using an eyepiece graticule. The trophozoites can be seen in a fresh saline preparation of stool, although accurate identification is possible on a permanently stained fecal smear. Entamoeba histolytica Introduction There is a large number of species of amoebae which parasitise the human intestinal tract. Of these Entamoeba histolytica is the only species found to be associated with intestinal disease (Fig. 1). Although many people harbour this organisms world wide, only about 10% develop clinically invasive disease, thus, the parasite has been shown to present two very different clinical presentations. 1. The commensal or non-invasive luminal form, where the parasite causes no signs or symptoms of disease. 2. The pathogenic or invasive form, where the parasite invades the intestinal mucosa and produces dysentery or amoebomas and may give rise to extraintestinal lesions via the blood, mainly to the liver. Sargeaunt and Williams (1978) conclusively proved that invasive and noninvasive strains of E. histolytica could be differentiated by isoenzyme electrophoresis, and the application of molecular biology has confirmed the presence of two distinct species with the same morphological features. The pathogenic or invasive species has retained the name E. histolytica, and the non-pathogenic, noninvasive species has been named E. dispar. Morphology of Trophozoites The trophozoites of E. histolytica / dispar, extracted from dysenteric stools, exhibit ingested red blood cells and clear pseudopodia. Those of E. dispar will have no ingested red blood cells. They can be up to 60µm in diameter, and their motility is rapid and unidirectional. On a permanently stained faecal smear e.g. Trichrome or Iron haematoxylin, the morphological features are more visible. When using Trichrome stain nuclei, chromidial bars, chromatin, red cells and bacteria stain red, cytoplasm stains blue-green and background and yeasts stain green. The presence of a small centrally placed karyosome is clearly visible. With Iron haematoxylin, nuclear chromatin and the karyosome will be stained immensely black. The remainder will be varying shades of grey/black. Morphology of cysts Cysts of E. histolytica / dispar are 10 – 15µm in diameter and contain 1 – 4 nuclei. Chromatoid bodies are usually present in young cysts as elongated bars with bluntly rounded ends. Glycogen is usually diffuse, but in young cysts it is often present as a concentrated mass, staining reddish brown with iodine. Clinical Signs of Disease Amoebiasis is an infection usually caused by the pathogenic Entamoeba histolytica / dispar, and is commonly an infection of the colon. It has a world wide distribution where environmental sanitation is poor. The parasite may behave as a commensal (causing no harm to the host) or it may act as a parasite (doing harm to the host). It is a disease of human beings, although some monkeys can become infected and the infection is then transmitted to humans. Intestinal Disease Patients with intestinal disease may exhibit a number of symptoms including profuse diarrhoea with blood and mucus, fever and dehydration. Amoebic ulcers may develop in the large colon and can also be found in the rectal area. The ulcers are usually "flask shaped" with a small opening on the mucosal surface and a larger area below the surface. Hepatic Disease Trophozoites are transported from the intestine to the liver, and liver disease is characterised with abdominal pain, fever, hepatomegaly and tenderness. If the abscess ruptures, it may spread to the brain, pericardium and other organs. If left untouched, the abscess will grow normally until it reaches the surface, where it can penetrate, e.g. the skin, the peritoneum, the pleural cavity or the pericardium. The dilatation of the liver is presumably the main source of the pain. Microscopy Where amoebic dysentery is suspected, the laboratory should be informed that a "hot stool" is being delivered so that it can be examined within twenty minutes. On cooling the amoebae stop moving and become very difficult to be identified. Direct microscopy should be done by mixing a small amount of the specimen in 0.9% sodium chloride solution. This permits detection of motile trophozoites of Entamoeba histolytica / dispar and can also provide information on the content of stool i.e. the presence of leucocytes and red blood cells. If one searchs e.g. primarily for cysts, not for amoebae, several stool samples are required to be examined, by direct microscopy and a sensitive concentration technique. Three negative stool samples are required before it can be found that there is no amoebic infection. Microscopic examination of an amoebic abscess aspirate, e.g. in the liver or lungs, may reveal haematophagous trophozoites. It must be examined immediately by mixing a drop of warm saline with some aspirated pus on a microscope slide. Serology If visceral or hepatic amoebiasis is suspected serological tests should be done, as microscopic methods do not always reveal characteristic trophozoites. The tests of choice are indirect fluorescent antibody test (IFAT), counter immunoelectrophoresis (CIEP) and enzyme linked immunosorbent assay (ELISA) The search for E. histolytica / dispar is mainly carried out in Europe and North America, as there is a natural concern to ensure that patients, even in the absence of symptoms are not harbouring parasites that may lead to serious complications later. Entamoeba coli Introduction Entamoeba coli is a non-pathogenic amoeba with world wide distribution. Its life cycle is similar to that of E. histolytica but it does not have an invasive stage and does not ingest red blood cells. Morphology of Trophozoite The trophozoite is larger than that of E. histolytica ranging from 15-50 µm in diameter. It exhibits blunt pseudopodia with sluggish movement. A permanently stained preparation shows a nucleus with a moderately large eccentric karyosome with chromatin clumped on the nuclear membrane. The cytoplasm appears to be granular containing vacuoles with ingested bacteria and other food particles. Morphology of Cysts Cysts of E. coli are 15 – 30 µm in diameter and contain 1 – 8 nuclei with irregular peripheral chromatin: karyosomes not central. Chromatoid bodies are not frequently seen but, when present, they are usually splinter-like with pointed ends. Glycogen is usually diffuse but in young cysts is occasionally found as a well-defined mass, which is stained reddish brown with iodine. Laboratory Diagnosis Laboratory diagnosis is made by finding the characteristic cysts in iodine stained, formol-ether concentration method or by detecting the characteristic trophozoites in a wet preparation or a permanent stained preparation. Entamoeba hartmanni Introduction Entamoeba hartmanni is a non-pathogenic amoeba with world wide distribution. Its life cycle is similar to that of E. histolytica but it does not have an invasive stage and does not ingest red blood cells. Morphology of Trophozoites Morphology of the trophozoites is similar to those of E. histolytica / dispar but they do not contain ingested red blood cells and their motility is less rapid. Morphology of Cysts Cysts of E. hartmanni 7-9 µm in diameter and contain 1 – 4 nuclei. Chromatoid bodies are usually present in young cysts as elongated bars with bluntly rounded ends. Glycogen is usually diffuse, but in young cysts it is often present as a concentrated mass, reddish brown in colour with iodine. Laboratory Diagnosis Laboratory diagnosis is made by finding the characteristic cysts in iodine stained, formol-ether concentration method or by detecting the characteristic trophozoites in a wet preparation or a permanent stained preparation. Endolimax nana Introduction Endolimax nana is a small non-pathogenic amoeba with world wide distribution. Its life cycle is similar to that of E. histolytica but is non-invasive. Morphology of Trophozoite Trophozoites of E. nana measure from 6-12 µm. Motility is sluggish with blunt hyalin pseudopodia. In a permanently stained preparation, the nucleus exhibits a large karyosome with no peripheral chromatin on the nuclear membrane. Morphology of Cysts Cysts of E. nana are 6 – 9 µm in diameter. They can be spherical or ovoid in shape and contain 4 pinpoint nuclei, which are seen by addition of iodine. Chromatoid bodies are not found and glycogen is diffuse. Laboratory Diagnosis Laboratory diagnosis is made by finding the characteristic cysts in iodine stained, formol-ether concentration method, or by detecting the characteristic trophozoites in a wet preparation or a permanent stained preparation. Entamoeba gingivalis Entamoeba gingivalis is an Entamoeba histolytica-like amoeba that lives in/on the teeth, gums, and sometimes tonsils. It measures 10-35 micrometers in length. Endocytotic vacuoles are often numerous and the parasite will ingest bacteria, leukocytes, and erythrocytes (dark circles in trophozoites, above) although it is not itself invasive. No cysts are formed and transmission is entirely by oral-oral contact. Multiple samplings reveal the parasite to colonize the oral cavity of nearly all adult humans. TOPIC: ZOOMASTIGOPHOREA Giardia lamblia (giardiasis) Introduction Giardia lamblia is a flagellate of world wide distribution. It is more common in warm climates than moderate climates. It is the most common flagellate of the intestinal tract, causing Giardiasis (Fig. 2). Humans are the only important reservoir of infection. Infection is most common in parts of the world where sanitation levels are the lowest. Giardiasis is an infection of the upper small intestine, which may cause diarrhoea. Only Giardia spreads disease. Morphology of Trophozoites The trophozoites of G. lamblia are flattened pear shaped and are of an average size of 15 µm long, 9 µm wide and 3 µm thick. When stained, the trophozoite is seen to have 2 nuclei, 2 slender median rods (axostyles), and 8 flagella arising from the anterior end. They have been described as looking like tennis rackets without the handle (they are often seen having a comical face-like appearance when observing at the front view). The movement of the trophozoites is described as tumbling leaf motility, using their 4 pairs of flagella for locomotion. They attach themselves to the surface of the jejunal or duodenal mucosa by their disc-like suckers, which are found on their ventral surface. They multiply in the gut by binary fission. Once the trophozoites drop off the mucosal surface they are normally carried in the intestinal contents down the gut where they usually encyst. Morphology of Cysts The cysts of G. lamblia are 8 – 12 µm in length and are elliptic in shape. They contain 4 nuclei, which tend not to be obvious. Longitudinal fibrils consisting of the remains of axonemes and parabasal bodies may also be seen. Cysts may appear to shrink from the cell wall. Cysts are infective as soon as they are passed. Clinical Signs of Disease Giardia lamblia colonizes the small intestine where the trophozoites adhere to the mucosal surface by means of their sucking discs. Cysts are produced as the parasites descending the intestinal tract, although trophozoites can pass in feces in severe infections. G. lamblia is transmitted through ingestion of cysts in contaminated water or food. Cysts can survive outside the body for several weeks under favourable conditions. The main symptoms are abdominal pain, flatulence, and episodic diarrhoea with steatorrhea and periodical soreness in severe cases. No blood or mucus is normally seen. Although, however, 50% of G. lamblia infections are symptomless, severe infections may develop in immunocompromised hosts. What determines susceptibility is poorly understood. After swallowing cysts for the first time, symptoms commonly develop 2-6 weeks later. Normally illness lasts for 1 to 2 weeks, but there are cases of chronic infections lasting months to years. Chronic cases, both those with defined immune deficiencies and those without, are difficult to treat. The disease mechanism is unknown, with some investigators reporting that the organism produces a toxin while others are unable to confirm its existence. The organism has been demonstrated inside host cells in the duodenum, but most investigators think this is such an infrequent occurrence that it is not responsible for disease symptoms. Mechanical obstruction of the absorptive surface of the intestine has been proposed as a possible pathogenic mechanism, as has a synergistic relationship with some of the intestinal flora. Thus, no matter what it looks like, stream water should be treated before drinking. Boiling will kill Giardia cysts, and there are commercially available filters that will remove the cysts from water. Laboratory Diagnosis Cysts can be found by examination of the deposit of a formol-ether concentrate of a stool preparation. The oval cysts with thick walls serve as characteristic features for these organisms. The flagella disintegrate and form a central “streak” which becomes visible when stained with iodine or MIF (merthiolate-iodine-formaldehyde). Cysts may be excreted intermittently; therefore it is important to examine more than one stool. Stools are usually passed 3-8 times / day and are usually pale, offensive, rather bulky and accompanied by much flatus. Trophozoites are found by examination of saline wet preparations of fresh, diarrhoeic stool, duodenal or jejunal aspirate or in a permanently stained fecal preparation. Trophozoites can also be found in the jejunal aspirate. These can be recovered by the String Test or Enterotest capsule and the material examined microscopically for motile trophozoites. Trophozoites and cysts can be found to be scarce in chronic infections. Serological methods of diagnosis are proved to be useful as means of diagnosis. An ELISA to detect IgM in serum provides evidence of a current infection. A polyclonal antigen-capture ELISA can be used to demonstrate submicroscopic infections in feces and an IgA-based ELISA will detect specific antibodies in saliva. Several strains of G. lamblia have been isolated and described through analysis of their proteins and DNA; type of strain, however, is not consistently associated with disease severity. Different individuals show various degrees of symptoms when infected with the same strain, and the symptoms of an individual may vary during the course of disease. Infectious Dose – Ingestion of one or more cysts may cause disease, as contrasted to most bacterial illnesses where hundreds to thousands of organisms must be consumed to produce illness. Trichomonas vaginalis (trichomoniasis, "trich" or "trick") Trichomonas vaginalis is a sexually transmitted disease (STD), although transmission by other routes (such as soiled towels) has been documented. There is no cyst in the life cycle, so transmission is via the trophozoite stage. Most people infected with trichomoniasis are asymptomatic. Symptomatic infections are characterized by a white discharge from the genital tract and itching. Diagnosis depends on finding trophozoites in secretions of the genital tract from men or women. In cases where the numbers of organisms are very low, the trophozoites can be cultured to increase their numbers. Trichomonas vaginalis, Pentatrichomonas hominis, Enteromonas hominis, and Retortamonas intestinalis Classification Protozoa. Phylum Sarcomastigophora. Flagellates. Disease Vaginal trichomoniasis in females and occasional symptomatology associated with infections of the prostate and epididymis of males (T. vaginalis); P. hominis, E. hominis, and R intestinalis are non pathogenic (Fig. 3). Geographic Distribution. Worldwide. Location in Host Mucosal surface of the vagina, the prostate gland and seminal vesicle (T. vaginalis), and intestinal tract (P. hominis, E. hominis, and R.. intestinalis). Morphology. Trophozoites Trophozoites of T. vaginalis are pyriform in shape, 7-30 μm long, with a width of 6-15 μm. Living trophozoites have jerky, nondirectional movement. They have four anteriorly directed flagella and the fifth one directed posteriorly along the outer margin of the undulating membrane; the latter extends only half the distance to the posterior end of the body. The nucleus is usually elongated in the anterior portion of the organism. The interior of the nucleus contains many chromatin granules and a small karyosome. The cytoplasm contains many dark-staining granules (hydrogenosomes), but these are not readily seen in Giemsa-stained specimens. A rodlike, pointed axostyle protrudes from the posterior end of the body. Pentatrichomonas hominis trophozoites are usually 6-14 μm long but some may be up to 20 μm long. They are morphologically similar to T. vaginalis except the posteriorly directed flagellum that forms the outer edge of the undulating membrane projects beyond the posterior end as a free flagellum. The axostyle is a slender rod extending from the anterior end through the middle of the body and projects from the posterior end. The anteriorly placed nucleus contains a small karyosome. Hydrogenosomes are readily visible in trichrome-stained organisms. Trophozoites of Enteromonas are usually 6-8 μm long but may be somewhat shorter or longer. They have three anteriorly directed flagella and the fourth one that extends posteriorly beyond the end of the body. They move in a rapid, jerky fashion. Their nucleus is near the anterior end and has a large, central karyosome. With fixation, trophozoites are spherical or elliptic and are somewhat smaller in size. Trophozoites of Retortamonas are ovoid or pyriform, 4-10 μm long by 3-7.5 μm wide, and have two flagella, one directed anteriorly and the other extending posteriorly. A cytostome is present at the anterior end; it extends posteriorly for nearly half the length of the organism and is bordered by a fibril. The nucleus is spherical, located in the anterior end, and it contains a small karyosome. There is a fine layer of peripheral chromatin. Cysts Trichomonas vaginalis and P. hominis lack a cyst stage. Cysts of E. hominis are small and inconspicuous, usually 4-8 μm long by 3-5 μm in width, and ellipsoidal in shape. Cysts will contain one, two, or four nuclei but binucleate forms predominate. When two nuclei are present, they frequently are at opposite ends; in those cysts with four nuclei, they are often paired at opposite ends. These nuclei have large, central karyosomes surrounded by a clear space; the nuclear membrane lacks peripheral chromatin. Cysts of Retortamonas are ovoid or pyriform in shape and measure 4-7 μm long by 3.5-4.5 μm in width. Mature cysts are uninucleate, have a compact central karyosome, and varying amounts of peripheral chromatin. The fibril associated with the cytostome in the trophozoite may be seen in the cyst, often near the nucleus. Life Cycle Trophozoite is directly transmitted during sexual intercourse (T. vaginalis) and presumably by ingestion of the trophozoite stage (P. hominis) or the cyst stage (Enteromonas and Retortamonas). Diagnosis The demonstration of trichomonads in vaginal secretions, scrapings, urethral discharge and sedimented urine from women, and in prostatic secretions and sedimented urine from men provide definitive diagnosis of T. vaginalis infection. In women, material for examination is best taken by using a platinum loop or cottontipped swab applied to the fornices of the vagina. A sample of scrapings from the loop is placed directly in a drop of saline solution on a slide, a cover glass is applied, and then the sample is examined by light, darkfield, or phase contrast microscopy for the presence of typical motile trophozoites. Organisms must be distinguished from squamous epithelial cells and polymorphonuclear leukocytes, which are also collected in the sampling procedure. Material taken by cotton swab should be agitated in a few drops of saline solution in a small test tube and the suspension then examined in a microscope slicie preparation. Fluorescence microscopy using vital dyes such as acridine orange has also been used but it is not apparent advantage over direct wet mount preparations. Giemsa-stained smears have been used when it is not feasible to examine wet mounts immediately. Trichomonads can be found in Papanicolaou-stained smears, but the morphology of the organisms is often altered by the staining procedure, making identification more difficult. Various culture media, including Diamond's medium, can be inoculated with material from the vagina, incubated anaerobically, and examined several days after inoculation for the presence of motile trophozoites. Commercially available culture systems have been developed which are useful to diagnose this infection. Also, urine samples can be sedimented and examined for the presence of organisms. The isolation of T. vaginalis from men is difficult and is best accomplished by examination of urine or prostatic secretions. Scrapings of urethral mucosa with a platinum loop and examination of this material as a wet mount preparation, or by inoculation of the material into culture media, are the usual procedures employed. If urine is examined, the sample should be taken at the first opportunity in the morning, preferably after prostatic massage; the sediment can be examined directly for the presence of organisms or it can be placed into culture media. The diagnosis of P. hominis, Enteromonas, and Retortamonas trophozoites in feces is best accomplished by direct wet mount preparations that reveal the typical jerky movement of these organisms. All of these organisms can be found in stained fecal smears; however, their affinities for stain are inconsistent. Diagnostic Problems Trophozoites of T. vaginalis frequently are difficult to find in wet mount preparations; however, the use of culture methods will increase the possibility of detecting an infection. A variety of staining methods have been used for demonstrating organisms. In Papanicolaou-stained smears, however, organisms are frequently distorted and difficult to identify; if a Gram stain is used, the organisms stain similarly to polymorphonuclear leukocytes and can be misidentified. In trichrome-stained fecal smears, P. hominis rarely stains well and is easily overlooked. The small size of both Enteromonas and Retortamonas results in these infections being rarely diagnosed or reported. The small size of E. hominis cysts leads to their being confused with Endolimax nana cysts. Comment Trichomonas vaginalis infection is an extremely important sexually transmitted disease throughout the world. In the United States, more than 1 to 2 million cases occur annually, and it is believed that on a worldwide basis there may be 100 to 200 million cases each year. Trichomonas hominis Introduction This flagellate is cosmopolitan in its distribution. It is thought to be nonpathogenic although it has been associated with diarrhoeic stools. It is the most commonly found flagellate next to G. lamblia and D. fragilis. It is found in a wide host range including non-human primates, cats, dogs and various rodents. Morphology of Trophozoites Trichomonas hominis does not have a cystic stage. The trophozoites measure from 5-15 µm in length by 7-10 µm in width. The shape is pyriform and has an axostyle which runs from the nucleus down the centre of the body and extends from the end of the body. They also possess an undulating membrane, which extends the entire length of the body and projects from the body like a free flagellum (this feature distinguishes it from other trichomonads). The characteristic number of flagella is five; there is some deviation from this number. They also have a single nucleus at the anterior end. Trichomonads swim with a characteristic wobbly movement, which makes diagnosis unmistakable. Laboratory Diagnosis In a fresh stool, the flagellates move very rapidly in a jerky, non-directional manner. The axostyle and undulating membrane are diagnostic. The flagellates are difficult to stain, however, the axostyle can be seen on a stained preparation and is diagnostic. Table 1 Differential morphology of flagellates found in stool samples of humans Species Size Shape (length) Pear Trichomonas 8-20 µm Usual range, Shaped hominis 11-12 µm Motility Nervous Jerky Chilomastix mesnili 6-24 µm. Pear Usual range, Shaped 10-15 µm Stiff Rotary Giardia lamblia 10-20 µm. usual range, 12-15 µm. Pear Shaped "Falling Leaf" Enteromona s hominis 4-10 µm. usual range 8-9 µm. Oval Jerky Retortamona s intestinalis 4-9 µm. Pear Usual range, Shaped 6-7 µm. or Jerky Number of Nuclei 1 Not visible in unstained mounts 1 Not visible in unstained mounts Number of Flagella 3-5 anterior, 1 posterior 2 Not visible in unstained mounts 1 Not visible in unstained mounts 4 lateral, 2 ventral, 2 caudal 1 Not visible in unstained 1 anterior, 1 posterior 3 anterior, 1 in cytostome 3 anterior, 1 posterior Other features Undulating membrane extending length of body Prominent cytostome extending 1/3 – 1/2 length of body. Spiral groove across ventral surface Sucking disc occupying 1/2 – 1/3 of ventral surface One side of body flattened. Posterior flagellum extends free posteriorly Prominent cytostome extending oval mounts approx 1/2 length of body * Not a normal feature for identifying species in routine stool samples Trypanosoma species Introduction Trypanosomes are haemoflagellates and three species of the genus Trypanosoma are responsible for disease in humans such as sleeping sickness. Trypanosomes occur in the blood of the majority of vertebrate animals. The life cycle involves intermediate host, which usually is an insect. Many species of trypanosomes can live in harmony with their hosts producing no pathogenic effect, but the best known species are those that are pathogenic to their definitive hosts. The disease caused by the pathogenic types is called trypanosomiasis. Salivarian trypanosomes Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense – These metacyclic trypanosomes are found in the proboscis of the insect vector – infection is therefore inoculative. The above are the aetiological agents of African trypanosomiasis, it is a zoonotic species in the fact that it multiplies in the blood of a range of animals, domestic and wild animals as well as a man. Trypanosoma brucei rhodesiense causes an acute form of sleeping sickness in East Africa, while T. b. gambiense causes chronic sleeping sickness in West Africa. These are known as salivarian trypanosomes as they complete their development in the salivary system (anterior portion of the vector). Transmission takes place by innoculation of the metacyclic stage. Stercorarian trypanosomes Trypanosoma cruzi – The metacyclic trypanosomes occupy a posterior position in the gut of the insect vector and are passed out in the faeces – infection is therefore contaminative. This is the aetiological agent of South American trypanosomiasis. These trypanosomes are known as stercocarian as they complete their development in the posterior region of the vector, so that the infective forms appear in the insects’ feces. Hosts are infected by a contaminative route. African Trypanosomiasis Life Cycle Transmission from one vertebrate to another is carried out by blood-sucking invertebrates, usually an insect. The vector for African Trypanosomes is the Tsetse fly, Glossina, and the species which cause the disease are T. b. gambiense (Fig. 4) and T. b. rhodesiense. Metacyclic (infective) trypomastigotes are inoculated through the skin when a tsetse fly takes a blood meal. The parasites develop into long slender trypomastigotes, which multiply at the site of inoculation where ulceration occurs. The trypanosomes continue to develop and then may invade the lymphatic tissue, the heart, various organs and in later stages, the central nervous system. Trypomastigotes are taken up by the tsetse fly (male and female) during a blood meal. The parasites develop in the midgut of the fly where they multiply. 2-3 weeks later the trypomastigotes move to the salivary glands transforming from epimastigotes into metacyclic (infective) trypomastigotes. The tsetse fly remains infective for all their life i.e. about 3 months. The mode of transmission mentioned above, metacyclic transmission, requires to be separated from mechanical transmission, a process in which trypanosomes survive, for a short time, on and about oral region of an insect and are inoculated into a new host when the vector bites again, without undergoing any developmental cycle. Metacyclic transmission requires a lapse of time to allow the trypanosomes to reach an infective stage by a particular developmental sequence in the vector, usually a period of several days. Morphology The parasite is an elongated cell with single nucleus, which usually lies near the centre of the cell. Each cell bears a single flagellum, which appears to arise from a small granule – the kinetoplast. The kinetoplast is a specialised part of the mitochondria and contains DNA. The length and position of the trypanosome’s flagellum are variable. In trypanosomes from the blood of a host the flagellum originates near the posterior end of the cell and passes forward over the cell surface, its sheath is expanded and forms a wavy flange called an undulating membrane. Development is characterised by the occurrence of three types of blood forms (polymorphic), these are: 1) Slender forms: long and thin, about 29m long, free flagellum. 2) Stumpy forms: thick and short, average length 18m, typically no free flagellum, but a short one may be present. 3) Intermediate forms: about 23m long with a moderately thick body and a free flagellum of medium length. Clinical Signs of Disease The early stages of African trypanosomiasis may be asymptomatic and there is a low grade parasitiaemia. This period may last for several weeks to several months. The disease may terminate untreated at this stage or go on to invade the lymph glands. Invasion of the lymph glands is usually accompanied by a high irregular fever with shivering, sweating and an increased pulse rate. The lymph glands near the bite often become swollen, in T. b. gambiense the glands at the back of the neck and T. b. rhodesiense usually the glands under the jaw are affected (Winterbottoms sign). As the disease progresses oedema of the eyelids, face and sleeplessness are features along with increasing lethargy and listlessness. Trypanosomes may invade the central nervous system giving symptoms of meningoencephalitis, confusion, apathy, excessive sleeping and incontinence. At this stage, the cerebro-spinal fluid (CSF) usually contains mononuclear cells and a few trypanosomes may be detected. If untreated, character changes, mental deterioration and coma develop, finally resulting in death. Such signs are more commonly seen with gambiense than in rhodesiense in which patients often die before these symptoms develop completely. Laboratory Diagnosis of African Trypanosomiasis Laboratory diagnosis of African trypanosomiasis is by: 1. Examination of blood for the parasites 2. Examination of aspirates from enlarged lymph glands for the parasites 3. Examination of the CSF for the parasite 4. Detection of trypanosomal antibodies in the serum 1. Examination of blood a) Thick and thin blood films (thick and thin blood films are made and stained with Fields stain and examined as for malaria parasites). b) Triple centrifugation technique. This method is carried out as follows: (І) 5 to 10 ml of citrated blood is centrifuged at 2000 rpm for 5 minutes to pack the red blood cells. (ІІ) The plasma and white cell layer are removed by a Pasteur pipette and transferred to a clean centrifuge tube. (ІІІ) This is centrifuged for a short time in order to deposit any red blood cells carried over. (ІV) The supernatant fluid is removed by pipette to a clean tube. (V) This is centrifuged at 5000 rpm for 10 minutes. (VІ) The supernatant fluid is removed with a pipette and discarded. (VІІ) The deposit is examined microscopically for trypanosomes. c) Miniature anion-exchange centrifugation technique (ref. Transactions Royal Society of Tropical Medicine and Hygiene. 1979. 73. 312-317) Heparinised blood is passed through an anion exchange column. As the blood travels down the column the red cells are adsorbed while the less strongly charged trypanosomes are washed through with saline. The eluate is centrifuged and examined microscopically for motile trypanosomes. d) Buffy coat examination. Trypanosomes are centrifuged in a microhaematoctit tube for 5 minutes. Parasites can be seen microscopically at the junction of the packed red cells and plasma. 2. Examination of lymph gland aspirates The aspirate can be examined microscopically by making a wet preparation, or if there is not much material, it can be dried on a slide and then stained with either rapid Field’s stain or with Giemsa and examined microscopically. 3. Examination of CSF In the late stages of African trypanosomiasis, trypanosomes may be found in the CSF together with IgM – containing morula (Mott) cells, lymphocytes and other mononuclear cells. Once the CSF has been collected it must be examined as soon as possible. The parasites are unable to survive for more than 15-20 minutes in CSF once it has been removed. The parasites become inactive, are rapidly lysed and will not therefore be detected. The CSF should be examined wet and spun down in a sterile tube and a film made from the deposit. The film is then stained with rapid Field’s or Giemsa and examined microscopically. NB. It is impossible to distinguish between T. b. gambiense from T. b. rhodesiense on a stained film, as the two subspecies, which infect man, are identical. South American trypanosomiasis Introduction Trypanosoma cruzi occurs throughout South and Central America, especially in Brazil, Argentina and Mexico causing the disease known as Chagas’ disease. It is estimated that over 24 million people are infected with this species. It is a zoonotic parasite with over 150 species of wild animals known to harbour the parasites, for example opposums, dogs, rates, pigs and cats (Fig. 5). It is transmitted to man by brightly coloured bugs belonging to the Reduviidae family, subfamily Triatominae. All stages of these bugs are known to become infected. The bugs live in the crack of the walls and vegetal roofs of the poorly maintained houses, coming out at night to feed on the exposed parts of the host’s body. Life Cycle Metacyclic trypomastigotes are deposited in feces on the skin as the triatomine bug (reduviid bug) feeds. The bug usually bites round the edges of the mouth and eyes. The trypomastigotes are either rubbed into the skin by scratching the irritated area or penetrate the conjunctiva or membranes of the nose and mouth. Trypomastigotes become amastigotes in localised reticular endothelial cells and multiply. The amastigotes develop into trypomastigotes, which are released into the blood when the cell ruptures. No multiplication of the parasite takes place in the blood in its trypomastigote stage. The trypomastigotes reach tissue cells especially heart muscle, nerves, skeletal muscle and smooth muscle of the gastrointestinal system through the blood and lymph. The trypomastigotes become amastigotes and multiply forming pseudocysts. Within the pseudocyst some amastigotes become elongated and develop first into epimastigotes and then trypomastigotes. When the cell ruptures the trypomastigotes are released into the blood and continue to circulate whilst others invade further tissue cells. The life cycle completes when a triatomine bug vector ingests circulating trypomastigotes. In the vector the trypomastigotes transform and develop into epimastigotes, multiply by binary fission in the gut of the bug. After about 10 – 15 days, metacyclic trypomastigotes are formed and can be found in the hindgut of the bug. Morphology Trypanosoma cruzi has a single form (monomorphic), about 20m in length, and characteristically curved. The kinetoplast is large, considerably larger than the Trypanosoma bruceii species already discussed. They sometimes appear as a bulge at the posterior end. The flagellum is medium in length. Clinical Signs of Disease Many people infected with T. cruzi remain asymptomatic and free from Chagas’ disease or experience only an acute infection without progressing to the chronic stage. The most severe form of the disease is most commonly seen in children younger than 5 years of age. Multiplication of T. cruzi at the site of infection can produce an inflamed swelling (chagoma) which persists for weeks. Trypomastigotes or amastigotes may be seen in the aspirate of the chagoma. Regional lymph nodes may become infected which frequently involve one side of the face. Unilateral oedema of the upper and lower eyelid may occur along with conjuctivitis. This is known as Romana’s sign. In the acute stage of infection trypomastigotes can be found in the blood. Symptoms may pass unnoticed, but there may be fever, malaise, increased pulse rate and enlargement of the lymph glands, liver and possibly spleen. Muscle aches and pains are characteristic at this stage and parasites may be seen in blood films. The acute form is most often seen in young children and occasionally can cause serious damage to the heart and other complications leading to death caused by central nervous system involvement. Chronic manifestations include signs of cardiac muscle damage with a weak and irregular heartbeat, oedema, heart enlargement leading to heart failure. Dilation of the digestive tract resulting in megaoesophagus and megacolon may also occur. About 10% of persons infected with T. cruzi develop chronic Chagas cardiopathy. Laboratory Diagnosis of South American trypanosomiasis is made by: 1. Examination of blood; 2. Xenodiagnosis; 3. Blood culture; 4. Serology. 1. Examination of blood a) Thick and thin blood films are made and stained with Fields stain and examined as for malaria parasites. Wet preparations of blood can also be examined for motile trypanosomes. b) Buffy coat examination – Trypanosomes are centrifuged in a microhaematoctit tube for 5 minutes. Parasites can be seen microscopically at the junction of the packed red cells and plasma. This technique is rapid and sensitive. Trypanosoma cruzi can often be seen in C, U or S shapes in stained films. 2. Xenodiagnosis Xenodiagnosis is useful in chronic and subacute (low parasitaemia) disease. Sterile bugs are fed on patients by attaching a black bag containing the bugs to the arm of the patient and allowing them to feed for 30 minutes. Twenty five to thirty days later the bugs are dissected and the contents of the hindgut and rectum are examined microscopically for the presence of trypanosomes. 3. Blood Culture Blood culture is as sensitive as xenodiagnosis but it requires sterile conditions. 4. Serology Serology tests include: (І) IFAT indirect fluorescence antibody test (ІІ) CFT complement fixation test (ІІІ) IHAT indirect haemaglutination test (ІV) ELISA enzyme linked immunoabsorbent assay Other lab findings include: Raised ESR, marked lymphocytosis with atypical mononuclear lymphocytes. NB. In certain areas of South America where Trypanosoma rangeli (non pathogenic species transmitted by Rhodnius bug) is found with T. cruzi all positive preparations should be checked to confirm T. cruzi. Leishmania species Introduction Leishmaniasis is caused by parasites of the genus Leishmania and is endemic in many parts of Africa, Asia and South America. It is transmitted by Phlebotomus species, sandfly. Leishmania species are mainly parasites of man and other animals, especially dogs and rodents. They cause diseases collectively known as Leishmaniasis; causing 3 types of disease i.e. visceral leishmaniasis, cutaneous leishmaniasis and mucocutaneous leishmaniasis. These are all debilitating and disfiguring diseases, which occur throughout the Old and New World. The parasites are unusual in that they live entirely within the cells of the reticulo-endothelial cells; they have become perfectly adapted as the proteolytic enzymes, which attack other foreign bodies in the blood stream, do not destroy them. Visceral leishmaniasis Human visceral leishmaniasis (VL), sometimes known as Kala-azar, is caused by Leishmania donovani complex; L. donovani and L. donovani infantum in the Old World and L. donovani chagasi in the New World (Fig. 6). The clinical features –azar caused by these species are similar, but they have different epidemiological features. The parent species L. donovani occurs in Asia (Northeastern China, India and Iran) and Africa (primarily Sudan, Kenya and Ethiopia) and can affect people of all ages. The parasite (L. d. infantum), which causes VL in countries bordering the Mediterranean, (Southern Europe as well as North Africa) affects young children as well as infants. It is now being seen in the immunocompromised. In the New World also, VL is mainly a disease of young children, with the causative organism L. d. chagasi being closely related to, but slightly different from, L. donovani. The main geographical foci of VL in Latin America are in northern and northeastern Brazil. Small foci are found in northern Argentina, Columbia and Venezuela. Sporadic cases are found in Central American countries, including Mexico. Cutaneous leishmaniasis Cutaneous leishmaniasis is caused by L. tropica, L. major and L. aethiopica in the Old World and L. mexicana complex in the New World (Fig. 7). Leishmania tropica is widely distributed around the Mediterranean basin, Afghanistan, Kenya, Armenia, Azerbaijan, Turkmenistan and Uzbekistan. Leishmania aethiopica is seen in the highlands of Ethiopia and L. major occurs in the Middle East, West Africa, North Africa and Kenya. Leishmania mexicana complex is found in Central America and the Amazon Basin. Mucocutaneous leishmania It is caused by the L. braziliensis complex and is found in Brazil, Eastern Peru, Bolivia, Paraguay, Ecuador, Columbia and Venezuela (Fig. 8). Life cycle All forms of infection starts when a female sandfly (Phlebotomus species) takes a blood meal from an infected host. Small amounts of blood, lymph and macrophages infected with Leishmania amastigotes are ingested. Once ingested the amastigotes transform to promastigotes in the sandfly, the non-infective promastigotes divide and develop into infective metacyclic promastigotes. These are formed in the midgut of the sandfly and migrate to the proboscis. When the sandfly bites the extracellular inoculated promastigotes at the site of the bite is phagocytosed by macrophages. After phagocytosis, transformation to dividing amastigotes occurs within 24 hours. Reproduction at all stages of the lifecycle is believed to occur by binary fission. No sexual stage has been identified. Morphology Leishmania exist as flagellated extracellular promastigotes in the Sandfly vector and as a flagellar obligate intracellular amastigotes within mononuclear phagocytes of their vertebrate hosts. The various species are not distinguishable morphologically from one another. When stained with Romanowsky stains such as Giemsa, amastigotes appear as round or oval bodies ranging from 2 – 3m in diameter with a well defined nucleus and kinetoplast, a rod shaped specialized mitochondrial structure that contains extranuclear DNA. The flagellated promastigote is spindle shaped, measuring 10 – 20m in length, not including the length of the flagellum. As in the amastigote form a nucleus and kinetoplast are clearly visible. Clinical Signs of Disease – Visceral Leishmaniasis The incubation period of VL may vary between 2 weeks and 18 months. The onset of VL is usually insidious with fever, sweating, weakness and weight loss. The most prominent findings are fever, hepatosplenomegaly and anaemia. The sites mainly affected are the liver, spleen and bone marrow. Enlargement of the liver is due to hyperplasia of Kupffer cells which are packed with amastigotes. The bone marrow is infiltrated with parasitised macrophages. Some organs, notably the kidneys, may show pathological changes secondary to deposition of immune complexes. In advanced cases, ascites and oedema can develop. Deaths are usually due to secondary bacterial infections such as pneumonia, tuberculosis or dysentery. Laboratory Diagnosis of Visceral Leishmaniasis 1. Microscopy Parasites may be found in a splenic aspirate, liver biopsy or bone marrow biopsy. These techniques, especially splenic aspirate and liver biopsy can be hazardous and require previous expertise in the procedure. a) Air dry smears. b) Fix in methanol for 1 minute c) Stain with Giemsa 1 in 10 in buffered distilled water pH 6.8 for 30 minutes (or use the rapid Field’s stain) d) Wash the slide in buffered water and drain dry Amastigotes of leishmania should be seen in positive smears. They are approximately 2-4µm in size, oval and are frequently seen within the cytoplasm of the macrophage. The amastigotes possess a nucleus and a rod – shaped kinetoplast within the cytoplasm. In many samples a very small number of parasites are present. Extensive searching of the film is necessary. 2. Culture The aspirates can be cultured in Novy-Nicolle-MacNeal (NNN) or Schneider's Drosophila medium. In culture the amastigote stage converts to the promastigote stage. However, this is not a rapid technique, as the parasites may take anything from 10-21 days to grow. 3. Serodiagnosis VL produces large amounts of specific IgG, which can be used for diagnosis. Currently the most used sero diagnostic tests are Indirect-immuno Fluorescent Antibody Test (IFAT), Enzyme Linked Immunosorbent Assay (ELISA) and Direct Agglutination Test (DAT). Clinical signs of disease – Cutaneous Leishmaniasis Following a bite from an infected sandfly, a small red papule appears at the site of the bite about 2-8 weeks later. The papule increases in size centrifugally. The patient then mounts either a hypersensitive response or an anergic response. In a hypersensitive response, the papule eventually ulcerates, becomes depressed and then eventually heals through scarring. The patient is now immune from subsequent bites. In an anergic response, the nodule grows and spreads over large areas of the skin. This resembles leprosy. Laboratory Diagnosis of Cutaneous Leishmaniasis 1. Slit skin smear. The margin of the lesion contains amastigotes whereas the centre contains debris and dead skin material. This margin of the lesion is aseptically punctured with a hypodermic needle and syringe containing a small amount of saline. The aspirate, which is drawn up into, the needle is examined microscopically and/or cultured using the method described in visceral leishmaniasis. 2. Polymerase chain reaction Gene amplification techniques are powerful and sensitive methods and are useful in diagnosis of cutaneous leishmaniasis, particularly when organisms cannot be detected microscopically. It is also very useful for the specification of Leishmania parasites, thus the correct treatment can be administered. Clinical sign of disease – Mucocutaneous Leishmaniasis Mucocutaneous leishmaniasis or espundia initially develops like cutaneous leishmaniasis but develops into lesions in the mucocutaneous junction of the pharynx resulting in the break down of the palate of the mouth and nose or more rarely the genitalia or anus. This occurs from a few weeks to several years after the cutaneous lesion has healed. These lesions result in disfiguring deformities of the nose and mouth. Laboratory Diagnosis of Mucocutaneous leishmaniasis 1. Microscopy Finding the organisms in a histological section of the lesion provides definitive diagnosis of mucocutaneous leishmaniasis. However, the organisms are very rare in this form of the disease and culture can be a more sensitive method (see visceral leishmaniasis). 2. Polymerase chain reaction PCR method has the advantage of not only low numbers of parasites in aspirates but also histological sections. This makes it a very sensitive method in diagnosing mucocutaineous leishmaniasis when parasites are difficult to be detected. TOPIC: APICOMPLEXA, SPOROZOAE, CILIOPHORA Toxoplasma gondii Introduction Toxoplasma gondii, the causative organism of toxoplasmosis, was first observed in 1927 in the gondii, a North African rodent. The first human case of toxoplasmosis was also reported that year. The organism is a coccidian protozoa belonging to the subphylum Apicomplexa and has a world wide distribution occurring in all warm-blooded animals. Cats are the definitive hosts and they become infected by ingesting oocysts or cysts in tissues of paratenic hosts, such as mice, or transplacentally. Man becomes infected either by direct ingestion of oocysts from a cat or by eating raw or undercooked meat. Those who handle raw meat are particularly at risk. Infection can be transmitted transplacentally. Life cycle The development of the entereoepithelial (sexual) cycle in the cat’s intestine is brought about by the ingestion of sporulated oocysts of a mouse with cysts. The prepatent period up to the shedding of the oocysts varies with the stage of T. gondii ingested, for example only 3-10 days if the cat has ingested a mouse containing cysts, but about 19-20 days or longer after direct infection with oocysts or ingestion of a mouse containing only tachyzoites. Women most at risk of delivering an infected infant are those who acquire the infection just prior to gestation (Fig. 9). Humans can acquire infection by: 1) Accidental ingestion of the oocyst shed in the cat’s feces; 2) Ingestion of the tachyzoite in infected milk or transplacentally; 3) Ingestion of the tissue cyst in undercooked or raw meat; 4) Transplant of an infected organ in a seronegative recipient. Clinical Signs of Disease Serological evidence has shown that approximately one third of the world's population has Toxoplasma antibodies. This suggests that the majority of infections are benign with most people exhibiting few or no symptoms, but fever and swelling may be seen. However Toxoplasma gondii can cause severe illness in congenital infections, acquired infections and in immunocompromised patients. This may lead to ocular toxoplasmosis and ultimately to fatal CNS disorders such as encephalitis. Congenital toxoplasmosis This occurs approximately in 1 per 1000 pregnancies. It can cause severe damage to and even death of the fetus. Proliferation of tachyzoites leads to intracellular calcification, corioretinitis, hydrocephaly, psychomotor disturbances and convulsions. A small proportion of babies who are asymptomatic at birth develop retinocoroiditis or mental retardation becoming children or young adults. When a mother is first exposed to the parasite in later pregnancy the infant is likely to be less severely damaged or asymptomatic. Acquired infections Infections with T. gondii are often mild with flu-like symptoms, thus they often go unnoticed. However lympadenopathy is the most easily recognized symptom and it can be accompanied by fever, headache and myalgia. Toxoplasmosis may also produce infectious mononucleosis-like symptoms. Ocular toxoplasmosis is also a common sign, however, it is not yet proved whether this is due to congenital or acquired infections. Other manifestations of Toxoplasma infections are meningoencephalitis, hepatitis, pneumonitis and myocarditis. Immunocompromised patients Toxoplasmosis has been shown to occur as an opportunistic pathogen in immunocompromised patients and can cause severe complications. Toxoplasmosis in immunocompromised patients almost always arises from a reactivation of latent infections. Conditions, which can predispose to toxoplasmosis are malignancies, organ transplants, leukaemias and patients with acquired immune deficiency syndrome (AIDS). In immunocompromised patients, the central nervous system is primarily involved with diffuse encephalopathy, meningoencephalitis or cerebral mass lesions. Toxoplasma encephalitis has been reported as a life threatening among patients with AIDS. Laboratory Diagnosis 1. Serological Techniques The detection of toxoplasma specific antibodies is most commonly used in clinical laboratories. Specific IgG antibodies typically persist for life, whereas specific IgM antibodies begin to decline after several months. Most laboratories make preliminary tests for IgG antibodies and more definitive tests including IgM and IgA are made in reference laboratories. The Sabin-Feldman Dye Test is the gold standard for detecting the presence of specific antibodies. It measures the total amount of specific antibody in the serum, which is capable of participating in antibody-mediated killing of tachyzoites by complement. This test involves the use of live tachyzoites, which are derived from infected mice or rats. Because of the use of live organisms, this test is not recommended in the use of routine laboratories and is, thus, only employed in consulting centres. 2. Isolation Techniques Culture of parasites in animals is the best overall method but it can take up to six weeks before the result is available and, thus, is a disadvantage. Tissue culture is taken more often three or four days to obtain a result, but is not as sensitive. 3. Antigen detection The direct detection of very small amounts of specific nucleic acid has been made possible by the introduction in 1985 of the polymerase chain reaction (PCR). This technique results in the amplification of a specific fragment of DNA from within the parasite genome, which is detected by ethidium bromide staining, following gel electrophoresis. PCR is so sensitive that it should detect Toxoplasma DNA in one cyst. However, this may indicate a latent infection rather than an active infection. Its sensitivity may create problems since it will detect very small amounts of DNA from latent as well as active infections and it does not differentiate between cyst and tachyzoite DNA. Thus, samples like blood, CSF, urine and amniotic fluid should be used as they do not contain the latent stages. PCR is much promising but yet it is still intensive and expensive for routine use in the laboratory. Malaria Blood Parasites Red blood cells offer parasites an excellent environment for invasion and survival. Haemosporina are the only protozoan parasites, which can invade the red blood corpuscles of vertebrates. Most of them, if not all, have multiplicative phases in the reticulo-endothelial system. The red blood cells are thin-walled and constantly moving, with the result that absorption of food materials and elimination of waste products of metabolism are relatively easy to achieve. In addition, they also contain rich supplies of protein and oxygen. Malarial parasites are known not actually to penetrate the red blood cell but, in fact, enter the cell membrane by endocytosis and enclosed in a parasitophorous membrane. Malaria Introduction Malaria is the most important tropical disease known to mankind, causing many deaths and much morbidity throughout the world. It remains a significant problem in many tropical areas especially in sub-Saharan Africa. In many areas of the world we have the situations, deteriorating as a result of environmental changes, including global warming, civil disturbances, increasing travel and drug resistance. (Greenwood, B.M, 1997) There are approximately 100 million cases of malaria worldwide with about 1 million of these proving fatal. Malaria is caused by protozoa of the Plasmodium species. There are 4 species which infect both humans and aniamls; Plasmodium malariae (quartian malaria), Plasmodium vivax (benign tertian malaria), Plasmodium falciparum (malignant tertian malaria, subtertian malaria) and Plasmodium ovale (ovale tertian malaria). The transmission of the protozoa, Plasmodium requires two hosts, an intermediate invertebrate host (vector), and a definitive vertebrate host (mammals, birds and lizards). All Plasmodium species undergo the general haemosporina developmental cycle, which can be summarised as: 1. Initial or continual schizogny in the vertebrate host with initiation of gametogeny; 2. Formation of gametes in the arthropod host and subsequent fertilization and formation of a zygote; 3. Formation of sporozoites from the zygote by repeated nuclear division followed by cytoplasmic divisions. (Smyth, J.D, 1994) There is no requirement for resistant stages since the transfer of parasites between the vertebrate and invertebrate hosts is made by withdrawal or injection during the bloodsucking act, there is little or no exposure to the hazards of the outside world; thus by blood transfusion or inoculation, via the blood stages of the parasite. Malaria is transmitted by the female anopheline mosquito. The life cycle of all species of human malaria parasites is essentially the same (Fig. 10). It comprises an exogenous sexual phase (sporogony) with multiplication in certain Anopheles mosquitoes and an endogenous asexual phase (schizogony) with multiplication in the vertebrate host. The latter phase includes the development cycle in the red cells (erythrocytic schizogony) and the phase taking place in the parenchyma cells in the liver (pre-erythrocytic schizogony). When a female Anopheles mosquito bites an infected person, it ingests blood, which may contain the mature sexual cells (male and female gametocytes), which undergo a series of developmental stages in the stomach of the mosquito. Exflagellation occurs resulting in the production of a number of male and female gametes. Fertilisation occurs producing a zygote, which matures to an ookinete. This penetrates into the stomach wall of the mosquito where it grows into an oocyst and it further matures to become a motile sporozoite. The length of the developmental stage in the mosquito not only depends on the Plasmodium species but also the mosquito host and the ambient temperature. This may range from 8 days in Plasmodium vivax to 30 days in Plasmodium malariae. The sporozoites migrate from the body cavity of the mosquito to the salivary glands and the mosquito now becomes infective. Sporozoites enter the blood stream of a host when the mosquito feeds on blood. Following the inoculation, the sporozoites leave the blood within 40 minutes and enter the parenchymal cells of the liver (hepatocytes). In all 4 species, asexual development occurs in the liver cells, a process known as pre-erythrocytic schizogony, to produce thousands of tiny merozoites, which are relaeased into the circulation after about 16 days. However, in P. vivax and P. ovale some sporozoites differentiate into hypnozoites, which remain dormant in hepatocytes for considerable periods of time. When they are “reactivated” they undergo asexual division and produce a clinical relapse. In P. falciparum and P. malariae hypnozoites are not formed and the parasite develops directly into pre-erythrocytic schizonts. Once getting the circulation, the merozoites invade the red cells and develop into trophozoites. In the course of their development they absorb haemoglobin of the red cells leaving as the product of digestion a pigment called haemozoin, a combination of haematin and protein. This iron-containing pigment is seen in the body of the parasite in the form of dark granules, which are more obvious in the later stages of development. After a period of growth the trophozoite undergoes an asexual division, erythrocytic schizogony. When the mature trophozoite starts to divide in the red blood cell, separate merozoites are formed resulting in a schizont. When fully developed, the schizont ruptures the red blood cell containing it, liberating the merozoites into the circulation. These merozoites will then infect new red cells and the process of asexual reproduction in the blood tends to proceed. Some of the merozoites entering red blood cells do not form trophozoites and then schizonts, but develop into gametocytes and this process takes place in deep tissue capillaries. This erythrocytic cycle of schizogony is repeated over and over again in the course of infection, leading to a progressive increase of parasitaemia. Infection with all four strains of malaria has many clinical features in common. These are related to the liberation of fever-producing substances, especially during schizogony. The common features are: fever: often irregular. The regular pattern of fever does not occur until the illness has continued for a week or more. It depends on synchronised schizogony; anaemia: anaemia is haemolytic in type. It is more severe in infections with P. falciparum because in this infection cells of all ages can be invaded. Also, the parasitaemia in this infection can be much higher than in other malarias; splenomegaly: the spleen enlarges early in an acute attack of malaria. When a patient has been subjected to many attacks, the spleen may be of an enormous size and lead to secondary hypersplenism; jaundice: mild jaundice due to haemolysis may occur in malaria. Severe jaundice only occurs in P. falciparum infection, and is due to liver involvement. Specific Characteristics of Species Plasmodium falciparum Introduction Plasmodium falciparum is the most important malaria parasite, found in the tropics and sub-tropics, being responsible for approximately 50% of all malaria cases. The incubation period of P. falciaprum malaria is the shortest, between 8 and 11 days and has a periodicity of 36 – 48 hours. It can be differentiated from the other species by the morphology of different stages found in the peripheral blood. In infections with Plasmodium falciparum usually only young trophozoites and gametocytes are seen in peripheral blood smears, the schizonts are usually found in capillaries sinuses of internal organs and in the bone marrow. The disease runs an acute course and often has lethal outcome. It is a significant cause of abortions and stillborns and even death of non-immune pregnant women. Life cycle The aspects of the life cycle, which are specific to P. falciparum, are as follows: a) It attacks all ages of erythrocytes so that a high density of parasites can be reached quickly. In extreme cases up to 48% of the red blood cells can be parasitised. b) Multiple infections resulting in several ring forms in a corpuscle are not uncommon. c) The latter stages in the asexual cycle do not occur in the peripheral blood as in other forms of malaria, so that only rings and crescents are found in blood films. After 24 hours the ring forms and older trophozoites show a tendency to clump together and adhere to the visceral capillary walls. They become caught up in the vessels of the heart, intestine, brain or bone marrow in which the later sexual stages are completed. d) Sporulation is not as well synchronised as in other malaria forms, so that the fever may last longer. e) Exo-erythrocytic forms do not persist in the tissues and hence relapses do not occur. Morphology of Trophozoites Red blood cells in Plasmodium falciparum infections are not enlarged and they may have a spiky outline, common in cells, which have dried slowly. The typical arrangement of cytoplasm in young trophozoites is the well-known ring formation, which thickens and invariably contains several vacuoles as the trophozoite develops. Chromatin is characteristically found as a single bead, but double beads and small curved rod forms frequently occur. Maurer’s dots are slow to appear and are first seen as minute purplish dots, 6 or less in number. The points become spots, still few in number and are now characteristic enough to be recognised. Maurer describes them as fine ringlets, loops or streaks. They are seldom absent from the red blood cells containing large rings but the staining of the spots is very sensitive to pH and is seldom seen if the pH falls below 6.8. Trophozoites of P. falciparum can be found on the edge of the red blood cells. These are known as acole forms and are found as three distinct types: 1. Common: The single chromatin bead lies on the edge of the cell with most of the cytoplasm extended along the edge on both sides of the bead. 2. Rim: The complete parasite lies in a thickened line along the edge of the cell with no evidence of ring formation. 3. Dispalced: The parasites are displaced beyond the edge of the host cell. All degrees of displacement may occur, from partial to marked displacement with most of the parasite lying beyond the cell margin. Gametocytes Gametocytes are the sexual stage of the malaria parasite. Plasmodium falciparum gametocytes appear in the peripheral circulation after 8 – 11 days of patent parasitaemia and they have assumed their typical crescentic shapes. They soon reach their peak density, and then decline in numbers, disappearing in about 3 months as a rule. The female form, or macrogametocyte, is usually slenderer and somewhat longer than the male, and the cytoplasm takes up a deeper blue colour with Giemsa stain. The nucleus is small and compact, staining dark red, while the pigment granules are closely aggregated around it. The male form, or microgametocyte, is broader than the female and is more inclined to be sausage shaped. The cytoplasm is either pale blue or tinted with pink and the nucleus, which stains dark pink, is large and less compact than in the female, while the pigment granules are scattered in the cytoplasm around it. In humans, gametocytes neither multiply, nor cause symptoms but they are the forms, which are infective to the mosquito. When a female Anopheline mosquito takes a blood meal, the male and female gametocytes continue their sexual development. Schizonts Schizonts are rarely seen in the peripheral blood and their presence may indicate a potentially serious parasitaemia. Schizonts have 8 – 36 merozoites and a large mass of golden brown pigment (haemozoin) seen in the pre-schizont and schizont stage. Clinical Signs of Disease Symptoms include headache, photophobia, muscle aches and pains, anorexia, nausea and vomiting. Complications include severe anaemia cerebral malaria, renal disease, black water fever, dysentery, pulmonary oedema and tropical splenomegaly syndrome. Plasmodium vivax Introduction Plasmodium vivax is found almost in all places, where malaria is endemic and is the most predominant of malaria parasites. Causing 43% of all cases of malaria in the world, it also has the widest geographical distribution. Although the disease itself is not usually life threatening, it can cause severe acute illness. Plasmodium vivax does not infect West Africans due to the fact that West Africans do not possess the Duffy Antigen on the red blood cells, which the parasite requires to enter the red blood cell. It has an incubation period of between 10 and 17 days, which is sometimes prolonged to months or years due to the formation of hypnozoites. It has a periodicity of 48 hours. Plasmodium vivax infections are usually characterised by the presence of more than one developmental stage in the peripheral blood film. The parasites parasitise young enlarged erythrocytes and Schüffner’s dots develop on the erythrocyte membrane (Fig.11). Life cycle The aspects of the life cycle, which are specific to P. vivax, are as follows: a) The degree of infectivity is low, only the young immature corpuscles are infected; about 2% of erythrocytes are parasitised. b) The periodicity of the asexual cycle is closely synchronised. c) Hypnozoites develop in the liver, so that relapses may occur. Morphology of Trophozoites Most trophozoites of P. vivax are already several hours old when they appear in peripheral blood and by that time the Schüffner’s dots are already visible. The trophozoites are actively amoeboid and contain single or sometimes double chromatin dots that are either circular or ovoid. As the trophozoites mature, the Schüffner’s dots increase in number and size and the parasite changes from large irregular rings to rounded or ovoid forms in mature trophozoites. Gametocytes Mature female gametocytes are large rounded parasites, which fill or nearly fill the host cell. The cytoplasm is blue and fairly homogenous. The nuclear chromatin is a single, well-defined purplish mass, varied in form and usually peripheral in distribution. Mature male gametocytes can be distinguished from females by the large, loose and ill-defined mass of chromatin and by their paler colour and smaller mass. Schizonts The parasitised red cells are much enlarged containing Schüffner’s dots. The parasites are large, filling the enlarged red cell. There are between 12-24 merozoites in the schizonts (usually16). The pigment is a golden brown central loose mass. Clinical Signs of Disease Symptoms include headache, photophobia, muscle aches and pains, anorexia, nausea and vomiting. Complications due to P. vivax are relatively rare and arise due do a previous debility or pre-existing disease. Plasmodium ovale Introduction Plasmodium ovale is widely distributed in tropical Africa especially the west coast, despite that it is a species that is rarely encountered. It has also been reported in South America and Asia. It has an incubation period of 10 – 17 days, which is sometimes prolonged to months or years due to the formation of hypnozoites. It has a periodicity of 48 hours; the fever it produces is milder than the benign tertian P. falciparum. Life cycle The features of the life cycle, which are specific to P. ovale, are as follows: a) It morphologically resembles P. malariae in most of its stages; b) The changes produced in the erythrocytes in general are similar to those produced by P. vivax, but Schüffner’s dots appear considerably earlier (in the ring stage) and are coarser and more numerous; c) In the oocyst the pigment granules are (usually) characteristically arranged in two rows crossing each other at right angles; d) Hypnozoites develop in the liver so that relapses may occur. Morphology Parasites of P. ovale are usually found in enlarged and stippled red blood cells (James’s dots), similar to those found in P. vivax infections. Host cells show an oval shape, particularly those containing younger stages of the parasites and the host cell may also show “spiking” or fimbriation. Trophozoites Young trophozoites are found as compact rings in cells containing Schüffner’s dots. The trophozoite rings remain compact as they develop and show little of the amoeboid features common in P. vivax. Small, scattered pigment granules can be seen in developing trophozoites, which disperse as the trophozoite matures. Late trophozoites are round and consolidated with an increase in cytoplasm, they are very similar to P. vivax at this stage. Gametocytes The mature gametocytes are round, filling two thirds of the red cell. The red blood cell is slightly enlarged and stippled and contains pigment, which has a distinct arrangement of concentric rods, mostly at the periphery. Schizonts The parasite is smaller than red blood cells and contains 6-12 merozoites, usually 8 in a single ring. The pigment is a brown / greenish central clump. The red cell is slightly enlarged, stippled, frequently oval and fimbriated. Clinical Signs of Disease Symptoms, like those of P. vivax, include headache, photophobia, muscle aches and pains, anorexia, nausea and vomiting. Complications due to P. ovale are relatively rare and arise due do a previous debility or pre-existing disease. Plasmodium malariae Introduction Plasmodium malariae occurs mainly in the subtropical and mild areas where P. falciparum and P. vivax occur. However, it is less frequently seen, responsible for approximately 7% of all malaria in the world. It has an incubation period of 18 – 40 days and a periodicity of 72 hours. Life cycle The features of the life cycle, which are specific to P. malariae, are as follows: a) Infected erythrocytes are not larger than uninfected ones and sometimes even smaller; b) Mature erythrocytes are attacked and rarely reticulocytes, so that the density of parasites is very low; about 0.2% of erythrocytes are parasitised; c) It is often difficult to distinguish between a large trophozoite and an immature gametocyte. Morphology Parasites of P. malariae are typically compact heavily pigmented parasites, which are usually smaller and more deeply stained than normal. They tend to parasitise small, old red blood cells, they do not contain any inclusion dots and the parasitaemia is usually low. Trophozoites Trophozoites are found as fairy large fleshy rings with a single chromatin dot. These can be much distorted and can often take the form of bands across the cell. All trophozoites have a single chromatin dot and contain pigment. Gametocytes Gametocytes contain large amounts of black pigment, with chromatin present as a compact mass in females and diffuse in males. They occupy less than two thirds of the red blood cell. Schizonts Schizonts are usually few in numbers with 6 – 12 large merozoites in a single ring. Pigment is usually present as a central black mass. The parasites present are generally only found at one stage of schizogony development. Clinical Signs of Disease Symptoms include headache, photophobia, muscle aches and pains, anorexia, nausea and vomiting. Plasmodium malariae, like P. vivax and P. ovale are relatively benign. However, chronic infections in children may lead to nephrotic syndrome due to immune complexes depositing on the glomerular wall. Diagnosis of malaria parasites Introduction The definitive diagnosis of malaria infection is still based on finding malaria parasites in blood films. In thin films the red blood cells are fixed so the morphology of the parasitised cells can be seen. Species identification can be made, based upon the size and shape of the various stages of the parasite and the presence of stippling (i.e. bright red dots) and fimbriation (i.e. ragged ends). However, malaria parasites may be missed on a thin blood film in case of low parasitaemia. Therefore, examination of a thick blood film is recommended. With a thick blood film, the red cells are approximately 6 – 20 layers thick which results in a larger volume of blood being examined. Thick Blood Films In examining stained thick blood films, the red blood cells are lysed, so diagnosis is based on the appearance of the parasite. In thick films, organisms tend to be more compact and denser than in thin films. Field’s stain method for thick blood films The method recommended for staining thick blood is Field’s Stain, which is made from 2 components. Field’s A is a buffered solution of azure dye and Field’s B is a buffered solution of eosin. Both Field’s A and B are supplied ready for use by the manufacturer. Method 1. Place a drop of blood on a microscope slide and spread to make an area of approximately 1 cm2. It should just be possible to read small print through a thick film. 2. The film is air dried and NOT fixed in methanol. 3. The slide is dipped into Field’s stain A for 3 seconds. 4. The slide is then dipped into tap water for 3 seconds and gently agitated. 5. The slide is dipped into Field’s stain B for 3 seconds and washed gently in tap water for a few seconds until the excess stain is removed. 6. The slide is drained vertically and left to dry. Microscopic features of the Field’s stained thick blood film a) The end of the film at the top of the slide when it was draining should be looked at. The edges of the film will also be better than the centre, where the film may be too thick or cracked; b) In a well-stained film the malaria parasites show deep red chromatin and pale blue cytoplasm; c) White cells, platelets and malaria pigment can also be seen on a thick film. The leucocyte nuclei stain purple and the background is pale blue. The red cells are lysed and only background stroma remains. The occasional red cell may fail to lyse; d) Schizonts and gametocytes, if present, are also easily recognizable; e) A thick film should be examined for at least 10 minutes, which corresponds to approximately 200 oil immersion fields, before declaring the slide negative. N.B. a) As a result of haemolysis of the red blood cells due to staining of an unfixed film, the only elements seen are leucocytes and parasites, the appearance of the latter being altered. Consequently: b) The young trophozoites appear as incomplete rings or spots of blue cytoplasm with detached chromatin dots. c) The stippling of P. vivax and P. ovale may be less obvious although occasionally ghost stippling may be seen. d) The cytoplasm of late trophozoites of P. vivax and P. ovale may be fragmented. e) Caution should be exercised when examining thick blood films as artefacts and blood platelets may be confused with malaria parasites. Thin Blood Films When examining thin blood films for malaria you must look at the infected red blood cells and the parasites inside the cells. 1. Rapid Field’s stain for thin films This is a modification of the original Field’s stain to enable rapid staining of fixed thin films. This method is suitable for malaria parasites, Babesia sp., Borrelia sp. and Leishmania sp. Method. 1. Dry the film 2. Fix in methanol for 1 minute. 3. Flood the slide with 1 ml of Field’s stain B, diluted 1:4 with distilled water. 4. Immediately, add an equal volume of undiluted Field’s stain A, mix well and allow to stain for 1 minute. 5. Rinse well in tap water and drain dry. Uses. This is a useful method for rapid presumptive species identification of malarial parasites. It shows adequate staining of all stages including stippling (mainly Maurer’s dots). However, staining with Giemsa is always the method of choice for definitive species differentiation. 2. Giemsa stain for thin films. Method. 1. Air dry thin films 2. Fix in methanol for 1 minute 3. Wash in tap water and flood the slide with Giemsa diluted 1:10 with buffered distilled water pH 7.2. The diluted stain must be freshly prepared each time. 4. Stain for 25 – 30 minutes. 5. Run tap water on to the slide to float off the stain and to prevent deposition of precipitate on to the film. Dry vertically. 6. Examine the film using the x100 objective. Microscopic features of the thin blood film 1. Examine the tail end of the slide where the red cells are separated into a one-celllayer thick. 2. An alkaline buffer pH 7.2 is vital for clear differentiation of nuclear and cytoplasmic material and to visualise inclusions such as Schüffner’s / James’s dots in the red cells. Acidic buffer is unsuitable. 3. The red cells are fixed in the thin film so the morphology of the parasitised cells and the parasites can be seen. 4. On a well stained film the chromatin stains red/purple and the cytoplasm blue. Leucocytes have purple nuclei, the red stippling, if present, should be clearly visible. Rings of the four main species of malaria may look alike. If you see rings, look for older stages. Patients with a P. falciparum infection only, rings are usually seen; older stages are present only in severe infections. In poorly stained slides, Schüffner’s dots may not be visible, so it is essential that correct staining methods are used. Also Schüffner’s dots may not be seen in the earlier rings of P. vivax or P. ovale. Estimation of Parasitaemia Percentage of Plasmodium falciparum Counting of red blood cells infected with parasites of P. falciparum is essential and parasitaemia percentage should always be reported as this has implications for prognosis and the pattern of treatment employed. The recommended procedure for estimating the percentage parasitaemia in a thin blood film is by expressing the number of infected cells as a percentage of the red blood cells e.g. 3 parasitised red cells / 100 red blood cells or 3% parasitaemia. A red blood cell infected with multiple parasites counts as one parasitised red cell. Parasitaemia percentage should be calculated by counting the number of parasitised red blood cells in 1000 cells in a thin blood film. Method 2 Alternatively the World Health Organisation recommends a method, which compares the number of parasites in a thick blood film with the white blood cell count. Parasitaemia is estimated by first counting the number of parasites per 200 white blood cells in a thick blood film and then calculating the parasite count / l from the total white blood cell count / l. Knowledge of either % parasitaemia or total parasite count is essential for the correct clinical management of P. falciparum malaria. The Intersep Malaria One Step Rapid tests are qualitative membrane based assays for the detection of malaria antibodies in serum, plasma or whole blood. Separate devices are available for the detection of Plasmodium falciparum and Plasmodium vivax antibodies. The device is precoated with the appropriate Malaria antigen on the test line and anti-conjugate antigen on the control line. During the test the sample is exposed to the conjugate complex. The sample migrates up the membrane binding with the test line in the presence of the malaria antibody. Independent of a positive reaction the sample continues to migrate to the control line where the conjugate binds to the membrane bound anti-conjugate antigen to demonstrate correct device function. A positive result is indicated by two lines and a negative result by the sole presence of the control band. Thin blood films for malaria diagnosis are best prepared from venous or capillary blood taken directly from the patient, without addition of anticoagulant. However, this is not usually possible in a clinical laboratory, as many samples are received from general practices and other hospitals. All anticoagulants have some effect on the morphology of malaria parasites and the red blood cell they inhabit. This effect depends on the stage of the parasite, the time taken for the blood to reach the laboratory and the type of anticoagulant used. If it is necessary to use an anticoagulant, the films should be prepared as soon as possible after the blood has been taken. If the films cannot be made immediately, potassium EDTA is the anticoagulant of choice. However if the blood is left for several hours in EDTA, the following effects may be seen. 1. Sexual stages may continue to develop and male gametocytes can exflagellate, liberating gametes into the plasma. These can be mistaken for organisms such as Borrelia. Gametocytes of P. falciparum, which have a characteristic crescent shape, may round up and then resemble those of P. malariae. 2. Acole forms, which are characteristic of P. falciparum, may be seen in P. vivax because of attempted reinvasion of the red blood cell by merozoites. 3. Mature trophozoites of P. vivax may condense when exposure becomes prolonged, and in cases of extreme exposure red blood cells containing gametocytes and mature schizonts may be totally destroyed along with the contained parasites. The malaria pigment, haemozoin, always remains and can provide a clue to the presence and to an expert eye identity of the parasite. 4. The morphology of the red blood cell may be altered by shrinkage or crenation. Table №2 Differential diagnostic features of human Plasmodia species – Giemsa stained thin film of peripheral blood Malaria species Red Cell Changes P. falciparum Maurer’s dots P. vivax Schüffner’s dots P. ovale James’s Dots / Fimbriation P. malariae Ziemann’s dots Trophozoite – Ring form Cytoplasm is very fine in young rings; thick and irregular in old rings. Acole forms, multiple infections common Red cell is unaltered in size, sometimes stippled with Maurer’s dots. Parasite is compact; pigment is dense brown or black mass Cytoplasm is fine in young rings. Red cell unaltered in size Cytoplasm is thicker than that found in P. vivax. Red cell unaltered in size Cytoplasm is noticeably thicker. Red cell unaltered in size Red cell is enlarged, stippled. Parasite – amoeboid, vacuolated; pigment fine and scattered, golden brown Red cell is unaltered in size, or slightly enlarged. Stippled; may be oval and fimbriated. Parasite – compact, rounded; pigment fine brown grains Red cell is unaltered Red cell is much Red cell is slightly Red cell is unaltered in size. Parasite – compact, ugly, rounded or band- shaped; dark brown / black pigment often concentrates in a line along one edge of the band Red cell is Trophozoite – Growing form Mature schizonts in size. Parasite – merozoites 8 – 36; pigment clumped, black. Rare in peripheral blood enlarged, stippled. Parasite – large, filling enlarged red cell. Merozoites 12 – 24, usually 16; pigment golden brown central loose mass Gametocytes Red cell is distorted. Parasite – crescentric. Rare in early cases < 10 days Red cell is enlarged, stippled. Parasite – large, fills red cell enlarged, stippled, frequently oval and fimbriated. Parasite – smaller than red cell. Merozoites 6 – 12, usually 8 in a single ring; pigment, brown / greenish central clump Red cell is slightly enlarged, stippled. Parasite – round, filling 2/3 of the red cell unaltered in size. Parasite – fills red cell. Merozoites 6 – 12, usually 8, sometimes forming a rosette; pigment, brown / black central clump Red cell is unaltered in size. Parasite – small, round, fills the red cell The Ciliates The ciliates belong to the family Ciliophora. They possess simple cilia or compound ciliary organelles, 2 types of nuclei and a large contractile vacuole. The only member of the ciliate family to cause human disease is Balantidium coli. Balantidium coli Introduction Balantidium coli is widely distributed in warmer climates, where human infections most commonly occur. The organisms inhabit the large intestine, caecum and terminal ileum where they feed on bacteria. The most common hosts are humans, pigs and rodents. Human infection is usually transmitted from pigs and is rare. Figure 12 illustrates the life cycle of Balantidium coli. Morphology of Cyst The cyst is spherical or ellipsoid and measures from 30-200 m by 20-120 m. It contains 1 macro and 1 micronucleus. The cilia are present in young cysts and may be seen slowly rotating, but after prolonged encystment, the cilia disappear. Cysts are formed when diarrhoea subsides, and the rectal contents become formed. The cyst, ingested by a fresh host, excysts to liberate the trophozoite. Morphology of Trophozoite Trophozoites of B. coli have approximately 30-150 m in length x 25-120 m in width, but have been known to attain lengths of up to 200 m. They are oval in shape and covered by short cilia. A funnel shaped cytosome can be seen near the anterior end. Multiplication is by binary fission in the trophozoite stage. In an unstained preparation, the organisms are easily recognised because of their size and rapid revolving rotation. In a stained preparation, the characteristic macro and micronuclei may be observed. Clinical Signs of Disease Severe B.coli infections may resemble amoebiasis. Symptoms include diarrhoea, nausea, vomiting and anorexia. The diarrhoea may persist for long periods of time resulting in acute fluid loss. Balantidium coli also has the potential to penetrate the mucosa resulting in ulceration just as those of Entamoeba histolytica, but perforation is more common. Metastatic lesions do not occur. Extra-intestinal disease has also been reported, but is rare. Laboratory Diagnosis Wet preparations of fresh and concentrated stool samples reveal the characteristic cysts and motile trophozoites. They are easier to identify in directsmear saline preparations than permanently stained fecal smears. TOPIC: PLATHELMINTHS, TREMATODA Helminth Parasites The word “worm” is used loosely to describe organisms with elongated bodies and a more or less creeping habit. The word “Helminth” does mean “worm”, but in zoological terms it is more restricted to members of the phyla Platyhelminths, Nematoda and Acanthocephala. There are three groups of medically important helminthes: Cestodes (tapeworms), Nematodes (roundworms) and Trematodes (flukes). These parasites live in both the body spaces (gut lumen, bile ducts, lungs, oral cavity, etc.) and in tissues (blood, muscles and skin). The Trematodes The trematodes (or flukes) are leaf shaped and can outer cover called the tegument, which may be smooth or spiny. There are 2 suckers or organs of attachment, an anterior oral sucker and a posterior ventral sucker. The suckers form a characteristic feature of the group, from which the name Trematode is derived from (trematodes is a Greek word for meaning holes). They can occur in a variety of host environments, with the majority being endoparasitic but some are found to be ectoparasitic. Most trematodes are hermaphroditic and most of their body consists of the reproductive organs and their associated structures. The digestive system is well developed; they generally feed on intestinal debris, blood, mucus and other tissues, depending on the host environment. Trematodes require an intermediate host in their life cycle with vertebrates being the definitive host. Larval stages may occur in either invertebrate or vertebrate hosts. There are three groups of trematodes: the Monogenea, which typically are external parasites of fish with direct life cycles. The Aspidogastrea, these are endoparasites with the entire ventral surface as an adhesive organ. Finally the third group is the Digenea; these are endoparasites with simpler adhesive organs and life cycles involving one or more intermediate hosts (indirect life-cycle). This section concentrates on the Digenean trematodes. Most digenean trematodes inhabit the alimentary canal of vertebrates and many of the associated organs, such as the liver, bile duct, gall bladder, lungs, bladder and ureter. These organisms are founf mostly in cavities containing food such as blood, mucus, bile and intestinal debris. The digenean trematodes have a complex life cycle, with rare exceptions, always involve a mollusk host. There may be six larval stages – the miracidium, sporocyst, redia, cercaria, mesocercaria (rare) and the metacercaria (the majority have 4 or 5 stages). Trematode eggs have a smooth hard shell and the majority of them are operculate. Intestinal and Liver Flukes: Gastrodiscoides hominis, Nanophyetus salmincola, Fasciolopsis buski, and Fasciola hepatica Classification. Helminths. Phylum Platyhelminths. Trematodes. Diseases Paramphistomiasis (Gastrodiscoides). Nanopbyetus infection, fasciolopsiasis (Fasciolopsis). Fascioliasis or sheep liver fluke infection (Fasciola). Geographic Distribution Gastrodiscoides are parasites of pigs and humans in India, Southeast Asia, and parts of the former USSR; N. salmincola is found in North America. Fasciolopsis occurs in China, Taiwan, Thailand, Indonesia, India, Bangladesh, and possibly Cambodia, Myanmar and Vietnam. Fasciola hepatica has a worldwide distribution, especially in sheep-breeding countries. Location in Host Gastrodiscoides occurs in the cecum and ascending colon; both Nanophyetus and Fasciolopsis live attached to the wall of the small intestine; and Fasciola lives in the bile ducts of the liver. Morphology. Adult Worms Gastrodiscoides adults are thick-bodied, pinkish in color, and measure 5-14 mm long by 5-8 mm in width. The body is pyriform with a small, conical anterior portion and a larger, discoidal posterior part that is concave ventrally. Adults of Nanophyetus are small, pyriform flukes measuring 0.8-1.1 m in length. Fasciolopsis buski adults have a large, broad, fleshy body measuring 20-75 mm long and 20 mm wide. The anterior end is rounded, not cone-shaped. Although the reproductive organs are extensively branched, the esophagus and intestinal ceca are not. The smaller branched ovary is situated anterior to the paired, large, dendritic testes. Fasciola hepatica adults are large, broadly flattened, fleshy worms measuring up to 30 mm long and 13 mm wide. The anterior end is distinctly cone-shaped. All of the internal organs, including the esophagus, intestinal ceca, and reproductive organs, are extensively branched. The paired testes lay one behind the other, posterior to the ovary. Eggs Unembryonaled eggs of Gastrodiscoides passed in feces measure 130-160 μm long and 62-75 μm in width and have a pale greenish-brown color. Eggs of Nanophyetus are light brown in color, broadly ovoid, and unembryonated when discharged in feces. They measure from 64-97 μm long and 34-55 μm wide, and have a bluntly pointed, thickened, and darkened area of the shell at the abopercular end. Eggs of Fasciolopsis and F. hepatica are similar in size and appearance, and are difficult to distinguish from each other. The operculated eggs are large, a light yellowish-brown, broadly ellipsoidal, and unembryonated when excreted in feces. They measure 130-150 μm by 63—90 μm. The operculum is small and indistinct. At the abopercular end of the shell of Fasciola eggs there is often a roughened or irregular area that is not seen in Fasciolopsis eggs. Life Cycles Although details of the Gastrodiscoides life cycle have not been clarified, it is believed that cercariae liberated from the snail intermediate host encyst to the metacercarial stage on aquatic vegetation. Nanophyetus salmincola infection is acquired by ingestion of infected fish that serve as the second intermediate host. The life cycles of Fasciolopsis and F. hepatica are similar (Fig. 13). Unembryonated eggs pass in feces into water where they become embryonated after 2 weeks or longer and infect appropriate snails. Cercariae emerge from these snails, attach to aquatic vegetation, such as watercress and water caltrop nuts, and undergo encystation to the metacercarial stage. Infection is acquired by ingestion of the metacercariae on this vegetation. Fasciolopsis buski matures directly in the duodenum in approximately 3 months. Larval F. hepatica migrate through the intestinal wall into the abdominal cavity, enter the liver, and burrow through the parenchyma to enter the bile ducts where the adult worms reach maturity in 3 to 4 months. It is not uncommon for these worms to end up in ectopic locations, including the abdominal wall, lungs, brain, and orbit because of the extraintestinal migration of the larval stages of Fasciola. Animals play a significant role in the life cycles of these flukes. Swine are the usual host for Gastrodiscoides. Many mammals and even birds are infected with Nanophyetus. In canids, an often fatal rickettsial disease known as salmon poisoning is carried by these flukes; this is particularly prevalent in the northwest United States and develops in dogs who have eaten raw infected salmon or other fish. Pigs are an important reservoir host for Fasciolopsis infection. Sheep and cattle are important hosts for F. hepatica infection, and in endemic areas economic losts of these animals may be severe. Diagnosis. Detection of characteristic eggs in feces. Diagnostic Problems Human Gastrodiscoides infection is uncommon but the large size of the eggs should aid in diagnosis. Nanophyetus salmincola infection principally has been reported from the northwestern United States, although people from eastern Siberia have a history of infection with different species of this parasite. Infection is associated with eating raw, incompletely cooked or smoked salmon and steelhead trout. Eggs of Nanophyetus must be distinguished from those of Diphyllobothrium latum, which they somewhat resemble in size and morphology. The similarity in size and morphology of the eggs of F. hepatica and Fasciolopsis may lead to difficulties in arriving at a correct diagnosis in areas of southeast Asia where these species overlap in geographic distribution. In such instances, clinical evaluation of the patient may be an important aid in diagnosis. Whereas F. hepatica is worldwide in distribution, a related species that infects herbivorous animals, Fasciola gigantica, can be found in the liver of humans in southeast Asia and Africa. The eggs of F. gigantica typically are larger (160-190 μm long by 70-90 μm in width) than those of F. hepatica and Fasciolopsis. The migration of F. hepatica and F. gigantica to ectopic locations in humans may be a feature of these infections that can pose a diagnostic problem. Spurious infections of F. hepatica and F. gigantica might be noted in individuals who have eaten the liver of infected cattle, water buffalo, or sheep. Comments Patients with Nanophyetus infection typically present with abdominal discomfort, diarrhea, and unexplained peripheral blood eosinophilia. Most infections with Fasciolopsis are light and asymptomatic, but in heavy infections, diarrhea and epigastric pain simulating a peptic ulcer are seen. In F. hepatica infection, extensive damage to the liver parenchyma because of the migration of immature flukes may occur. Acute Fasciola infections in children can be fatal. Fasciolopsis buski, and Fasciola hepatica Introduction Fasciola, Fasciolopsis and Echinostoma species are trematodes which parasitise the liver and intestines of a variety of vertebrates, they are hermaphroditic. Fasciola hepatica trematodes are not thought to infect man but in fact man is not an unusual host, with infections being reported in many countries including Europe and the USA. Eating of unwashed watercress appears to be the source of infection, with them ending up in the liver. The most common host is sheep where they can cause severe disease. Fasciolopsis buski (giant intestinal fluke) is a duodenal parasite infecting both man and pigs. They are found widespread in Asia and China, but they have been found to be endemic in Taiwan, Thailand, Bangladesh and India. Night soil is used as a fertilizer in these countries on plants such as water chestnut and caltrops. The snails graze on these crops and also the definitive hosts eat them raw and unwashed, peeling the edible water plants with their teeth. Infection with Echinostoma species is thought to be contracted by injestion of fresh water snails containing metacercaria, such as Echinostoma ilocannum which occurs in the Philippines. The metacercariae infect the large snail Piola luzionica and in return are eaten raw. Despite the large numbers of these flukes they are of little medical importance, the most important being F. buski. Table 3 Table describing the characteristics, differentiating the various Fasciola species, which are important to man Species Fasciola hepatica Fasciola gigantica Fasciolopsis buski Echinostoma species Geographic Distribution Cosmopolitan Reservoir Hosts Africa, the Orient and Hawaiian Islands Far-East and Indian Subcontinent South East Asia and Japan Camels, Cattle and Water Buffalo Pigs, Digs and Rabbits Sheep Variety of Mammals Location of adult Size of Ova in host Bile Ducts 130 – 150m by 63 – 90m Bile Ducts 160 – 190m by 70 – 90m Intestine 130 – 140m by 80 – 85m Intestine 88 – 116m by 58 – 69m Life cycle and transmission The life cycles of Fasciola, Fasciolopsis and Echinostoma species are complex, requiring more than one intermediate host. Adult worms inhabit the liver or bile ducts of the definitive host (human), where they lay many eggs, which are deposited into the environmnent in feces. They are immature when passed. If they are passed into water they become mature in 9 to 15 days at the optimum temperature of 22 – 25C. The larvae (miracidia) develop within the eggs and hatch out into water, where they penetrate the snail (intermediate host). The miracidia must find a snail within 8 hours for hatching out of egg. Each species of fluke favours one particular intermediate host; F. hepatica invade snails from the genus Lymnaea, the most important being Lymnaea truncatula and F. buski invade snails of the family Planorbidae, e.g. Segmentina hemisphaerula. Within snails the miracidia mature into sporozoites and then into redia and cercariae. This development takes 1 to 2 months depending on the temperature. (In summer there is only one redial generation but in cooler weather there are two redial generations.) The cercariae leave the snail, usually at night: they then swim around for several hours and then encyst (forming a resistant external wall) on submerged vegetation or fresh water fish. If the cysts are ingested by a definitive host, the metacercariae excyst in the duodenum. They then migrate through the intestinal wall and either reach the liver tissue and bile ducts via the body cavity or via the lymphatics and the circulation. They require 2 to 3 months to reach maturity. The life cycle of the Echinostomes differs by one minor point: the cercariae encyst withers within the tissues of the same molluscan host in which sporocysts and rediae develop, or penetrate and encyst in other animals such as amphibians or fish. Morphology The morphology of the adult flukes of Fasciola, Fasciolopsis and Echinostoma species is well documented. They are large leaf-shaped parasites about 2 –3 cm long. There are two suckers, an anterior oral sucker surrounding the mouth and a ventral sucker (acetabulum) on the ventral surface. The outer tegument is covered in tiny spines, which face backwards enabling them to attach themselves along with their suckers to the tissues. Ova are all thin shelled, ellipsoid, quinone coloured (bile stained) with an operculum that is often inconspicuous. Although ova of Echinostoma species can usually be differentiated by size due these flukes beign much smaller in size than F. Buski and F. hepatica, there is much cross-over in the size of Fasciola and Fasciolopsis species. Pathogenesis Light infections due to Fasciola hepatica may be asymptomatic. However, they may produce hepatic colic with coughing and vomiting; generalised abdominal rigidity, headache and sweating, irregular fever, diarrhoea and anaemia. Infections due to Fasciola gigantica occur mainly in cattle-breeding areas and cause clinical symptoms similar to those of Fasciola hepatica, although human infections are less common. The adult flukes of Fasciolopsis buski attach to the intestine, resulting in local inflammation and ulceration. Heavier infections may subsequently lead to abdominal pain, malabsorption and persistent diarrhoea, oedema and even intestinal obstruction. Marked eosinophilia may be seen. The adult flukes of Echinostoma species attach to the intestine resulting in little damage to the intestinal mucosa. Light infections are generally asymptomatic and heavy infections may produce light ulceration, diarrhoea and abdominal pain. Laboratory diagnosis Definitive diagnosis is made by observing the ova in feces; since the flukes are very prolific any significant infection will be easily picked up. In case if identification cannot be made from the size of the ova, clinical information and the source of infection may help to provide a diagnosis. Serological techniques are available for the diagnosis of Fasciola hepatica. The Intestinal Parasite IQA kit is a comprehensive kit containing stained protozoan slides and formalin fixed suspensions of helminth ova and larvae and a semi-interactive CD-rom (The CD-rom is an excellent form of interactive reference material, allowing you to understand easily the complex subject of parasitology). It is ideal for teaching, reference and internal quality assessments. Parasep fecal parasite concentrators are provided enabling concentrations to be carried out on the helminth suspensions. All reagents and products are supplied to prepare slides. Quality assessments can then be carried out following the staining procedures as recommended on the picture reference cards. Liver Flukes: Clonorchis sinensis, Opisthorchis species, and Dicrocoelium dendriticum Classification. Helminths. Phylum Platyhelminths. Trematodes. Diseases. Clonorchiasis Dicrocoeliasis. (Chinese liver fluke infection), Opisthorchiasis, Geographic Distribution China, Taiwan, Japan, Korea, Vietnam (Clonorchis); Thailand and Lao People's Democratic Republic (O. viverrini); Eastern Europe and former USSR (O. felineus); Europe, former USSR, northern Africa, northern Asia, areas of the Far East, and the western hemisphere (Dicrocoelium). Location in Host. Bile ducts of the liver. Morphology Adult Worms. Adult Clonorchis are flattened and spatulate, measuring 10-25 mm long and 3-5 mm wide. They are hermaphroditic, with a single, rounded ovary situated anterior to the two extensively branched testes. Adults of O. viverrini and O. felineus from humans are usually smaller, 7-12 mm long, and their slightly lobed testes are posterior to the oval or lobed ovary. Dicrocoelium adults measure 5-15 mm long and 1,5-2.5 mm in width, and their paired, slightly lobed testes are immediately behind the ventral sucker and anterior to the ovary. Eggs The ovoid, yellow-brown eggs of Clonorchis have a moderately thick shell and a seated operculum that results in its prominent "shoulders" appearance; they measure 27-35 μm and 12-19 μm. There is usually a small knob at the abopercular end. These eggs contain a miracidium when passed in feces. Eggs of O. viverrini and O. felineus are similar in morphology and size, measuring 19-29 μm long and 12-17 μm in width. Operculate Dicrocoelium eggs are thick-shelled, have a deep golden brown color, contain a miracidium, and measure 38-45 μm long and 22-30 μm in width. Life Cycle Clonorchis and Opisthorchis have similar life cycles involving appropriate freshwater snails as the first intermediate hosts and many fish as second intermediate hosts. Metacercariae encyst under the scales or skin of fish. Infection is acquired by ingestion of raw or inadequately cooked infected fish. Clonorchis metacercariae, ingested by a mammalian host, migrate to the bile ducts of the liver via the common bile duct and mature in 3 to 4 weeks. The life span of adult Clonorchis may be 20 to 25 years. Cats and dogs serve as animal reservoirs for human infection. Dicrocoelium has a markedly different life cycle that involves the use of appropriate terrestrial snails as first intermediate hosts. Large numbers of cercariae are released from snails and agglomerate within the mucus of molluscs to form "slime balls" that are deposited on soil or grass. These slime balls are ingested by ants that serve as the second intermediate host; infection is acquired by the ingestion of ants harboring metacercariae. Sheep, cattle, deer, and other herbivores are the usual hosts for this parasite, but human infections are common in many areas. Diagnosis. Detection of characteristic eggs in feces. Diagnostic Problems Clonorchis eggs sometimes are confused with heterophyid eggs but generally are somewhat larger and have a prominent seated operculum, whereas heterophyid eggs usually have an inconspicuous operculum flush with its shell surface. Although Clonorchis eggs typically have a knob or hooklike protrusion at the abopercular end, it is often difficult to see or may be absent. Although Opisthorcbis eggs are quite similar to those of Clonorchis, the shoulders of these eggs are not usually as prominent. Ingestion of infected livers from herbivorous animals may result in spurious passage of Dicrocoelium eggs in human feces. Comments Clonorchis infections occurring in residents of Hawaii and the west toast of the United States who have not been to the Orient probably are caused by eating of imported pickled fish containing still-viable metacercariae. In Canada, a related liver fluke, Metorchis conjunctus, has been found to cause clinical illness (abdominal pain, anorexia, and liver function abnormalities) in people eating raw fish, in particular the while sucker, Catostomus commersoni. Eggs of this parasite measure approximately 28 μm in length by 16 μm in width and are morphologically indistinguishable from the eggs of Opisthorchis species. Clonorchis sinensis Introduction Clonorchis sinensis, also known as the Chinese liver fluke is a narrow elongate liver fluke found in the Far East, mainly Japan, Korea, China, Taiwan and Vietnam. It belongs to the group of Oriental liver flukes where there are three main species, which commonly infect man. The other two species are Opisthorchis felineus and Opisthorchis viverrini. The three species are so similar in their morphology, life cycles and pathogenicity that they are very rarely discussed as separate species. All members of this group are parasites of fish-eating mammals, particularly in Asia and Europe. Man is the definitive hosts and water snails and fish are the intermediate hosts. Infections can be easily avoided by man not eating raw fish since this is the only way that infection can be passed on. Clonorchis sinenesis parasitise the biliary duct in humans who become infected by eating raw or undercooked fish. Dogs and cats are the most important reservoir hosts. Life cycle and transmission The adult flukes are found in the bile ducts and gall bladder where they deposit eggs. The eggs are passed out into the environment in feces (Fig. 14). Then they enter the gut in the bile. Further development can only take place if they are eaten by appropriate species of water snails (intermediate hosts), e.g. Bulimus fuchsianus. Within the snails body the miracidium (which hatched out of the operculated egg) matures into a sporocyst and then a redia (both are asexual replicative stages). Within the redia, several cercariae develop with unforked tails. These escape into the surrounding water when the redia finally bursts. They can live in the water for 1 – 2 days waiting to come in contact with a suitable species of fish (over 80 species have been recognised as susceptible hosts), they force their way in through the scales, lose their tails and encyst as infective metacercariae. Humans become infected when they eat raw or slightly pickled fish, the metacercariae excyst in the duodenum and descend the bile ducts. There they develop into adult flukes within 4 weeks. From infection of the snail to the formation of the infective metacercariae about 8 weeks pass. The ova of Clonorchis sinensis contain fully developed miracidia and possess prominent opercular shoulders (flask shaped egg) and are operculate. They are bile stained and measure 29m and 16m. In wet mounts they are transparent and you can quite easily see their anatomy. There can be up to 6,000 worms present and a daily egg output of 1,000 eggs per microlitre of bile or 600 per gram of feces. The cercariae possess eyespots; the penetration and cystogenous glands are also well developed. Pathogenesis Millions of people become infected every year but only a minority suffers from any illness. The pathology is related to the number of parasites present. Light infections of up to 50 eggs or more are usually asymptomatic. A heavy infection of 500 or more eggs may cause serious illness. Acute infections may be characterized by fever, diarrhoea, epigastric pain, enlargement and tenderness of the liver and sometimes jaundice. The invasion by these worms in the gallbladder may cause cholecystitis due to flukes becoming impacted in the common bile duct. Laboratory diagnosis Definitive diagnosis is made by observing the characteristic ova in feces following concentration of feces or from duodenal aspirates when there is complete obstructive jaundice or from the Entero-Test. Table 4 Table summarising the less common flukes that are known to infect man Heterophyes heterophyes Far East Geographic distribution Location of Small intestine adult in host Size of ova 26.5- 30m by 15 – 17m Shape of ova Prominent opercular shoulders Bile stained Eating raw or Infection pickled fish acquired by Occasionally Symptoms diarrhoea and vomiting Metagonimus yokogawai Far East Opisthorchis viverreni Thailand Dicrocoelium dendriticum Far East Small intestine Liver and bile ducts Liver and bile ducts 38 – 45m by 22 – 30m Dark brown, thick shelled and large operculum Eating infected ants Biliary and digestive problems 26.5- 30m by 15 – 26.7 by 15m 17m Prominent Prominent opercular opercular shoulders shoulders Bile stained Bile stained Eating raw or pickled fish Occasionally diarrhoea and vomiting Eating raw fresh water fish Malaise and right upper quadrant pain Metagonimus yokogawai Classification. Helminths. Phylum Platyhelminthes. Trematodes. Diseases. Metagonimiasis. Geographic Distribution Metagonimus yokogawai is found in China, Japan, south-eastern Asia, and the Balkan states. On a worldwide basis, these intestinal fluke typically have highly localized geographic distributions. Location in Host. This intestinal fluke lives in the crypts and lumen of the small intestine. Morphology. Adult Flukes M. yokogawai are minute, pyriform-.shaped organisms, measuring 1.0-2.5 mm long and 0.3-0.7 mm wide. Characteristically they have a fleshy collar at the anterior end that is provided with spines and partially surrounds the oral sucker. Eggs Eggs are small, ovoid, operculate, and yellow-brown, measuring 20-30 μm by 15—17 μm. They contain a miracidium when discharged in feces. With most of the genera listed above, the sizes of their embryonated eggs overlap considerably, generally between 20-30 μm in length. M. yokogawai eggs resemble the eggs of Fasciola and Fasciolopsis in shape but are considerably smaller, measuring 80-115 μm by 58-70 μm. These eggs are thin-shelled, unembryonated when laid, and have an inconspicuous operculum. Frequently, they have a roughening or slight thickening of their shell at the abopercular end. Life Cycles Intestinal flukes use fresh-water snails and fish as first and second intermediate hosts, respectively. In fish, the metacercarial stages typically are encysted under the scales or in the skin, and humans become infected by eating raw or inadequately cooked fish. In some instances, marine bivalves such as clams and oysters serve as intermediate hosts. The prepatent period is usually short, from 1 to 3 weeks, and the normal life span is up to several months. Diagnosis. Presence of characteristic eggs in feces. Diagnostic Problems Because the egg-laying capacity of M. yokagawai and other small intestinal flukes is limited, sedimentation concentration procedures may be needed to demonstrate eggs in light infections. Accurate species identification is often difficult because the eggs of most of these flukes are similar in size and morphology; without knowledge of the types of intestinal flukes occurring in the animals in any geographic area a specific identification is frequently impossible. Eggs of many of the intestinal flukes may be confused with those of the liver flukes, Cionorchis sinensis and Opisthorchis species. Usually the eggs of the liver flukes are somewhat larger (27-35 μm by 12-19 μm), have a seated operculum, and a knob at the abopercular end. Comments The presence of this infection in humans, as well as other infections caused by related, small intestinal flukes, are generally a result of the lack of host specificity by parasites. In a particular geographic region where fish are eaten raw or inadequately cooked, birds, rodents, dogs, cats, and other mammals often serve as reservoirs for human infections. In addition to the trematodes described above, numerous other small flukes of animals in south-eastern Asia occasionally have been reported from humans. Pathology caused by this small intestinal fluke is usually limited to mild inflammatory changes resulting from the attachment of the parasites to the mucosal epithelium. Mild, intermittent mucous diarrhea and colicky pain are often associated with these infections, but these symptoms are usually limited to a period of several months to 1 year, because of a short life span of the adult worms. Paragonimus westermanni Introduction Paragonimus westermanni is a lung fluke found in both humans and animals. The adults are 12mm long and are found in capsules in the lung. Although they are hermaphroditic, it is necessary for worms to be present in the cyst for fertilization to occur. The disease is seen in the Far East, China, south-eastern Asia and America. Life cycle Humans become infected by ingestion of insufficiently cooked crayfish or crabs containing metacecariae which excyst in the intestine, penetrate through the wall into the peritoneal cavity and make their way through the diaphragm and pleura into the lungs (Fig. 15). The lung cysts in which the worms most commonly occur usually contain 1 – 3 flukes. The eggs become freed into the bronchial tubes and pass out with sputum, but they may also appear in the feces in large numbers, as a result of being swallowed. Sporocyst and 2 redia generations occur, giving rise to creeping cercariae. These penetrate a number of fresh-water crustaceans, in which they encyst in various sites such as gills, muscles, heart and liver. Encysted metacercariae are not immediately infective but have to undergo further maturation. Mammalian hosts ingest the cysts, which are then ingested in the duodenum and the freed metacercariae penetrate through the intestinal wall into the body cavity to reach the pleural cavity in about 4 days and the lungs in about 14 – 20 days. In the lungs, a fibrous capsule is formed by the host, after about 6 weeks the worms mature and produce eggs, which characteristically appear in the sputum. Morphology The adult worm is an ovoid, reddish brown fluke about 12 mm long. The eggs are ovoid, brownish yellow, thick shelled and operculated. They measure 80 – 100m and 45 – 65m and may be confused with the ova of Diphyllobothrium latum. Clinical Signs of Disease As the parasites grow in the lung cyst, inflammatory reaction and fever occurs. The cyst ruptures and cough develops resulting in the increase of sputum. The sputum is frequently blood tinged and may contain numerous dark brown eggs and Charcot-Leyden crystals. Haemoptisis may occur after paroxysms of coughing. Dyspnoea and bronchitis develop with time. Bronchiectasis may occur and pleural effusion is sometimes seen. The disease resembles pulmonary tuberculosis. Cerebral calcification may also occur. Laboratory Diagnosis Diagnosis is based on finding the characteristic eggs in brown sputum. The eggs can also be found in the feces due to swallowing sputum. A chest X-ray may show cystic shadows and calcification. Serological tests, in particular, the ELISA method, are useful diagnostic tests. The Schistosomes Introduction The Schistosomes are blood trematodes belonging to the Phylum Platyhelmintha. They differ from other trematodes in that they have separate sexes. The male worms resemble a rolled leaf where they bear the longer and more slender female in a ventral canal (the gynaecophoric canal). They require definitive and intermediate hosts to complete their life cycle. There are 5 species of Schistosomes responsible for human disease; S. mansoni, S. haematobium and S. japonicum with S. mekongi and S. intercalatum being less common. They are the only trematodes that live in the blood stream of warm-blooded hosts. The blood stream is rich in glucose and amino acids, so along with the plasma and blood cells, it represents the environment, suitable for egg producing trematodes. Over 200 million people are infected over at least 75 countries with 500 million or more people exposed to infection. Improved water supplies promote spread of disease, forming potentially new habits for snails. The disease caused is called schistosomiasis or Bilharzia and is the most important of helminthic diseases. Infection by the three most common species is the same in both sexes and in all age groups. Though, S. mansoni and S. haematobium is seen to occur more often and most heavily in teenagers especially males. Life Cycle Adult worms of S. mansoni live in the plexus of veins draining the rectum and colon, and in branches of the portal vein in the liver. Adults of S. japonicum live in the anterior mesenteric blood vessels and in the portal vein in the liver. Whilst the adults of S. haematobium live in the vesical plexus draining the bladder. Once the eggs are laid by the adult female worms, the majority of them first pass through the veins of the blood vessel in which the worm is living, and then into the lumen of the intestine and pass in feces (S. mansoni and S. japonicum), or into the lumen of the bladder, and are then excreted in the urine (S. haematobium). Those eggs that reach fresh water hatch, releasing a miracidium, which must infect a snail of the correct species within 24 hours. The eggs of each species are markedly different but each produce virtually identical miracidium. Asexual multiplication takes place in the snail, and results in the release of cercariae (minute in size with forked tails, 200m long) into the water about 3 – 6 weeks later. Cercariae actively swim around and when they have located, or come into contact with a definitive host, they actively penetrate the skin. They can stay active looking for a host for 24 – 48 hours after which, if they don’t find a host, they will die. The head of the cercariae migrates to the liver and develops into either adult male or female worms (flukes), here they pair up and then migrate to their region of the venous blood system (species specific sites). The females leave the males and move to smaller venules closer to the lumen of the intestine or bladder to lay their eggs (about 6 weeks after infection). The majority of adult worms live from 2 – 4 years, but some can live considerably longer. Schistosoma mansoni Introduction S. mansoni occurs in West and Central Africa, Egypt, Malaysia, the Arabian Peninsula, Brazil, Surinam, Venezuela and the West Indies. The intermediate host is an aquatic snail of the geuns Biomphalaria. Man is the most common definitive host; occasionally baboons and rats are infected. The adult worms live in smaller branches of the inferior mesenteric vein in the lower colon. Morphology The adult males measure up to 15 millimetres in length and females up to 10 mm. The schistosomes remain in copula throughout their life span, the uxorious male surrounding the female with his gynaecophoric canal. The male is actually flat but the sides roll up forming the groove. The cuticle of the male is covered with minute papillae. The female only posses these at the anterior and posterior end as the middle section being covered by the male body. Oral and ventral suckers are present, with the ventral one being lager serving to hold the worms in place, preventing them being carried away by the circulatory flow (Fig. 16). The ova of S. mansoni are 114-175m long and 45-68m wide. They are light yellowish brown, elongate and possess a lateral spine. The shell is acid fast when stained with modified Ziehl-Neelsen Stain. A non-viable egg is dark coloured and shows no internal structural detail or flame cell movement. Eggs can become calcified after treatment and are usually smaller, appear black and often distorted with a less distinct spine. The schistosomes differ from other trematodes in that they are dioecious, digenetic, their eggs are not operculate and infection is acquired by penetration of cercaria through the skin. Clinical Signs of Disease The clinical sigs of disease are related to the stage of infection, previous host exposure, worm burden and host response. Cercarial dermatitis (swimmers itch) follows skin penetration and results in a maculopapular rash, which may last for 36 hours or more. After mating, the mature flukes migrate to the venules draining the large intestine. Their eggs are laid and they penetrate the intestinal wall. They are then excreted in feces, often accompanied by blood and mucus. It is the eggs and not the adult worms, which are responsible for the pathology, associated with S. mansoni infections. The adult flukes acquire host antigen, which protects them from the host's immune response. The host's reaction to the eggs, which are lodged in the intestinal mucosa, leads to the formation of granulomata and ulceration of the intestinal wall. Some of the eggs reach the liver via the portal vein. The granulomatous response to these eggs can result in the enlargement of the liver with fibrosis, ultimately leading to portal hypertension and ascites. The spleen may also become enlarged. Other complications may arise as a result of deposition of the eggs in other organs e.g. lungs. Katayama fever is associated with heavy primary infection and egg production. Clinical features include high fever, hepatosplenomegaly, lymphadenopathy, eosinophilia and dysentery. This syndrome occurs a few weeks after primary infection. Laboratory Diagnosis. Microscopy Laboratory confirmation of S. mansoni infection can be made by finding the eggs in feces. When eggs cannot be found in feces rectal biopsy can be indicated. Serology Serological tests are of value in the diagnosis of schistosomiasis when eggs cannot be found. An enzyme linked immunosorbent assay (ELISA) using soluble egg antigen, is employed at HTD. Schistosoma japonicum Introduction Schistosoma japonicum is found in China, Japan, the Philippines and Indonesia. It causes disease of the bowel with the eggs being passed out in feces (Fig. 17). It differs form S. mansoni and S. haematobium that is called zoonosis, in which a large number of mammals serve as reservoir hosts, cats, dogs and cattle play major roles in the transmission of the disease. The life cycle is not very different from that of S. mansoni, the intermediate hosts are from the subspecies Oncomelania hupensis. Sexual maturity is reached in about 4 weeks and eggs may be seen in the feces as quickly as 5 weeks after. They worms live coupled together in the superior, mesenteric veins and desposit 1500 – 3500 eggs per day in the vessels of the intestinal wall. The eggs infiltrate through the tissues and are passed in feces. Morphology The adult worms are longer and narrower than the S. mansoni worms. The ova are about 55 – 85m and 40 – 60m, oval with a minute lateral spine or knob. Clinical Signs of Disease The main lesions appear due to the presence of eggs, occurring in the intestine and liver. The eggs which are sequesters in the intestine mucosa or submucosa initiate granulomatous reactions, resulting in the formation of pseudotubercules. Due to the number of eggs released by the females the infection is more severe than one with S. mansoni. This is also happens due to the better adaptation of the parasite to man, therefore, the circumoval granuloma is very large. The initial illness can be prolonged and sometimes fatal. Laboratory diagnosis. Microscopy Laboratory confirmation of S. japonicum infection can be made by finding the eggs in feces. When eggs cannot be found in feces rectal biopsy can be indicated. Other Intestinal Schistosome species Other Schistosome species, which are responsible for human disease, are S. mekongi and S. intercalatum. These two species cause similar symptoms to that of S. mansoni and can be summarised in the table 5. Table 5 Table describing other less common intestinal Schistosome species that are known to cause disease in man S. mekongi S. intercalatum Geographic location Mekong River basin Central and west Africa Diagnostic specimen Stool, rectal biopsy, serology Stool, rectal biopsy, serology Egg size 30-55 by 50-65m 140-240 by50-85m Egg shape Oval, minute lateral spine or Elongate, terminal spine knob Schistosoma haematobium Introduction Schistosoma haematobium differs from the other two species previously mentioned and that it causes urinary schistosomiasis. It occurs in Africa, India and the Middle East. The intermediate host is the Bulinus snail. Just like S. mansoni, its distribution runs parallel to the irrigation projects and in areas, which favour the intermediate hosts. They are exclusively parasites of man. The mature worms live in copula mainly in the inferior mesenteric veins and the females deposit their eggs in the walls of the bladder and finally making their way into the urine. The life cycle is very similar to that of S. mansoni, with sexual maturity being reached within 4 – 5 weeks, but eggs may not appear in the urine until 10 – 12 weeks or even later (Fig. 18). Morphology The adult worms are longer than those of S. mansoni. The ova are relatively large, measuring 110m – 170m in length and 40m – 70m in width. They have an elongated ellipsoid shape with a prominent terminal spine. Clinical Signs of Disease The clinical signs of disease are related to the stage of infection, previous host exposure, worm burden and host response. Cercarial dermatitis (Swimmer’s Itch) following skin penetration results in a maculo-papular rash and can last for36 hours or more. The mature flukes of S. haematobium migrate to the veins surrounding the bladder. After mating, the eggs are laid in the venules of the bladder and many penetrate through the mucosa, enter the lumen of the bladder and are excreted in the urine accompanied by blood. Thus, haematuria and proteinuria are characteristic, though not invariable features of urinary schistosomiasis. As with all Schistosoma species, it is the eggs and not the adult worms which are responsible for the pathology associated with S. haematobium. In chronic disease, eggs become trapped in the bladder wall resulting in the formation of granulomata. Following prolonged infection, the ureters may become obstructed and the bladder becomes thickened resulting in abnormal bladder function, urinary infection and kidney damage. Chronic urinary schistosomiasis is associated with squamous cell bladder cancer. Heavy infections in males may involve the penis resulting in scrotal lymphatics being blocked by the eggs. Laboratory diagnosis The definitive diagnosis of urinary schistosomiasis is made by finding the characteristic ova of S. haematobium in urine. Terminal urine should be collected as the terminal drops contain a large proportion of the eggs. The urine can either be centrifuged and the deposit examined microscopically for ova. Eggs can sometimes be found in seminal fluid in males. A bladder biopsy is seldom necessary to make the diagnosis. A rectal snip may show the presence of ova as they sometimes pass into the rectal mucosa. Serological tests can be of value when eggs cannot be found in clinical samples. An enzyme linked immunosorbent assay using soluble egg antigen to detect antischistosome antibody is most sensitive. There is a marked periodicity associated with the time when most eggs are passed out. Higher numbers of eggs are encountered in urine specimens passed between 10 am and 2 pm, presumably as a result of changes in the host’s metabolic and physical activities. TOPIC: PLATHELMINTHS, CESTOIDEA Larval Cestodes, which Infect Man Infections in man with Echinococcus granulosus, Echinococcus multilocularis and Multiceps multiceps are caused by an accidental ingestion of eggs, which are excreted by the definitive animal host. The disease that developes due to the invasion of these parasites is caused by the larval stages or hydatid cyst, and is known as hydatid disease or hydatidosis. Each cestode possesses an elongated tape-like body, which lacks an alimentary canal. The adult tapeworms are strings of individuals having a complete set of reproductive organs (proglottids) in progressive degrees of sexual maturity and budding off from a body attached to the host tissue by a head or scolex. The larval stage, show a wide variation being found in almost any organ of both vertebrate and invertebrate hosts. Echinococcus granulosus Introduction Echinococcosis or Hydatid disease in man is caused by the larval stage of the dog tapeworm, Echinococcus granulosus. Hydatid disease is most extensively found in East Africa, North Africa, South Africa, the Middle East and parts of South America and Australia. The intermediate hosts are cattle, sheep, pigs, goats or camels and the definitive host for this disease is the dog or other canids. Life cycle of the cestode Larval infection in man causes hydatid disease. Adult worms are only seen in the definitive hosts, dogs, they cannot develop in man (Fig. 19). Man is an accidental intermediate host of hydatid disease. When the ova are ingested by a suitable intermediate host, they hatch in the duodenum and the oncosphere migrates to the blood stream where it is carried to the liver, lungs and other organs of the body. Here it develops into a hydatid cyst, which consists of an outer thick laminated cyst wall, and an inner, thin nucleated germinal layer. From the inner layer brood capsules are produced which contain protoscoleces. The brood capsules detach from the germinal layer, releasing free protoscoleces. Hydatid sand is the name given to the fluid in the cysts, which consists of protoscoleces, tissue debris, and sometimes free hooklets. Here, the life cycle stops in humans, but is continued when a hydatid cyst containing protoscoleces egg in sheep liver, is ingested by a suitable canine host where the protoscoleces develop into adult worms. Morphology The adult worm measures approximately 3 – 8.5 mm long. The scolex has 4 suckers and a rostellum with hooks, the latter becoming tightly inserted into the crypts of Lieberkühn. The mature strobila has only 3 – 4 proglottids, one is immature, one is mature and the final one is gravid; when gravid the eggs are expelled in the faeces. Due to the close similarity of the eggs to other Taenia species found in dogs they were until recently thought to be morphologically indistinguishable. The larvae in man develop into a unilocular cyst which gives rise to unilocular hydatid disease. This is characterised as having only one bladder or many completely isolated bladders, each enclosed in its own well-developed envelope. The latter consists of several layers, the most prominent being the laminated layer. Within this again is the germinal membrane from which the brood capsules arise inside and develop thousands of larvae or protoscoleces, the whole being suspended in a hydatid fluid. Clinical Signs of Disease Hydatid disease in humans is potentially dangerous depending on the location of the cyst. Some cysts may remain undetected for many years until they become large enough to affect other organs. Symptoms are then of a space occupying lesion. Lung cysts are usually asymptomatic until there is a cough, shortness of breath or chest pain. Hepatic cysts result in pressure on the major bile ducts or blood vessels. Expanding hydatid cysts cause necrosis of the surrounding tissue. Slow leakage of the hydatid fluid results in eosinophilia and rupture of an abdominal hydatid cyst results in severe allergic symptoms. Symptoms may not manifest themselves for 5 – 20 years after the infection. Laboratory Diagnosis 1. Imaging and serodiagnosis are the mainstay of diagnosis. Serological tests include Enzyme linked immunosorbent assay (ELISA), an indirect haemagglutination test a complement fixation test and a Western Blot system. 2. Microscopic examination of the cyst fluid to look for the characteristic protoscoleces, which can be either invaginated or evaginated. The cyst fluid will also reveal free hooklets and tissue debris. 1% eosin may be added to the fluid to determine the viability of the protoscoleces. Viable protoscoleces exclude eosin whereas nonviable protoscoleces take up the eosin. 3. Histological examination of the cyst wall after surgical removal. Western Blots One serological test, which is proved to be of value to diagnosing Hydatid disease, is the Western Blot. The test presents a definitive means for detection of human antibodies to the cestode E. granulosus. Diagnosis can be made using the Western Blot assay for the detection of IgG antibodies in serum reactive with E. granulosus antigens present on a membrane. Field studies support a sensitivity of 80% and specificity of 100% in patients with hepatic cysts. This assay is known as the Qualicode Hydatid Disease Kit, the principle behind the test is that it is a qualitative membrane-based immunoassay manufactured from E. granulosus proteins. The E. granulosus proteins are fractionated according to molecular weight by electrophoresis on a ployacrylamide slab gel (PAGE) in the presence of sodium dodecyl sulfate (SDS). The separated E. granulosus proteins are then transferred via electrophoretic blotting from the gel into strips for testing of individual samples. During the procedure, the strips containing the E. granulosus proteins are incubated with serum specimens and washed to remove unbound antibodies. Visualisation of human immunoglobulins specifically bound to E. granulosus proteins is performed by sequential reaction with goat anti-human immunoglobulinalkaline phosphatase conjugate and BCIP/NBT substrate. Bands corresponding to the positions of the resoled E. granulosus proteins will be visualized on the strip, indicating the presence in the serum sample of IgG antibodies directed against E. granulosus antigens. Band positions are compared to those on a reference strip developed using Hydatid disease positive control. Prevention 1. Safe disposal of dog feces. 2. Education to prevent feeding uncooked offal to dogs. Echinococcus multilocularis Introduction The larvae of Echinococcus multilocularis is a particularly dangerous species causing multilocular (alveolar) hydatid disease in man and animals and is common in the highlands of Europe i.e. Switzerland and Germany, in Canada, Alaska and Northern Russia. The most common definitive hosts are foxes and wolves in addition to domestic cats and dogs when they have access to infected rodents. Life cycle Foxes are the primary definitive hosts, although, under domestic circumstances dogs can act as the definitive host. Rodents are the intermediate hosts. Man is an accidental host by the ingestion of eggs where multilocular cysts are formed. In these cysts, the limiting membrane is thin, and the germinal epithelium may bud off externally resulting in proliferation in any direction. Metastases may occur. Unlike E. granulosus, there is little fluid in the cysts of E. multilocularis. Morphology The morphology is in general very similar to that of E. granulosus, but the adults are much smaller. Unlike E. granulosus, cysts of E. multilocularis in man do not contain daughter cysts with scolices. Instead, the larval cyst, or as it is referred to as an alveolar or multilocular hydatid cyst, forms a multicystic structure made up of proliferating vesicles embedded in a dense fibrous stroma, which is often mistaken for a hepatic sarcoma. In older cysts the hydatid fluid is replaced by a jelly-like mass. Clinical Signs of Disease Cysts are formed primarily in the liver and their growth in the vena cava or portal vein results in metastases in the lung or brain. Clinical signs of disease are similar to that of E. granulosus. Diagnosis 1. Laboratory diagnosis can be made by ELISA. 2. Clinical diagnosis is made by ultrasound. Table 6 Differences between the hydatid cysts of E. granulosus and E. multilocularis Echinococcus granulosus Echinococcus multilocularis Slow development of cyst Rapid development of cyst Cysts have thick-walled chambers Cyst has thin-walled chambers Separated by connective tissue Not separated by connective tissue Cyst is fluid filled Cyst is gelatinous filled Cyst is free of host material Cyst is contaminated by host material The Cestodes The cestodes (or tapeworms) form a group of worms, exhibiting two unmistakable morphological features; they all possess flat, ribbon-like bodies and lack an alimentary canal. Adult tapeworms usually inhabit the alimentary canal of their hosts (though they occasionally are found in the bile or pancreatic ducts) and attach themselves to the mucosa by means of a scolex. Despite the lack of the digestive system they do absorb food from the hosts intestine; thereby providing the tapeworms a habitat that is associated with high nutritional levels causing the tapeworms high growth rate. Larvae on the other hand show a wide range of habitat preferences, being found in almost any organ of both vertebrate and invertebrate hosts. Though, most larval species show a preference for a particular site. This lack of the alimentary canal markedly separates tapeworms from nematodes and trematodes. The outer tegument of the body must serve not only as a protective coating but also as a metabolically active layer through which nutritive material can be absorbed, along with secretions and waste material to be transported out of the body. The body consists of a chain of segments or proglottids, which can be immature, mature or gravid; the latter contains a fully developed uterus packed with eggs. Therefore, each tapeworm is made up of a ‘string of individuals’ having a complete set of reproductive organs in progressive degrees of sexual maturity and budding off from a body attached to the host tissue by a head or scolex. Except for Hymenolepis nana, which can develop directly in the same host, the lifecycle of tapeworms involves both an intermediate and definitive host. Taenia species Introduction Taenia species are the most common cestode parasites of humans. More than 60 million people are infected with T. saginata (“beef” tapeworm) world-wide and about 4 million are infected with T. solium (“pork” tapeworm). T. saginata has a comsmopolitan distribution, but is more common in developing countries where hygiene is poor and the inhabitants have a tendency of eating raw or insufficiently cooked meat. T saginata is the most common adult tapeworm found in man. T solium is virtually extinct in Europe and the USA. The adults of both species live in the small intestine of man, the definitive host (Fig. 20). The gravid segments are very active and escape through the anus, releasing large numbers of eggs in the perianal region or on the ground where they can survive for long periods. When ingested by pigs or cattle, the eggs hatch, each releasing an oncosphere, which migrates through the intestinal wall and blood vessels to reach striated muscle within which it encysts, forming cysticerci. When inadequately cooked meat containing the cysts is eaten by man, the oncospheres excyst, settle in the small intestine and develop there into adult cestodes over the next 3 months or so. The segments of T. solium are somewhat less active than those of the beef tapeworm but its eggs, if released in the upper intestine, can invade the host (auto-infection), setting up the potentially dangerous larval infection known as cysticercosis in muscle of any other site. (Peters & Gilles, 1995) Both humans and cattle or pigs are necessary to the complete life cycle of Taenia species. (In Europe and the USA cattle are the normal intermediate hosts, but in the tropics several other ruminants, e.g. goat, sheep, llama and giraffe, may serve as the intermediate hosts.) Eggs ingested by the intermediate hosts usually contain oncospheres. The oncospheres then hatch out in the duodenum, pass into the intestine where they penetrate the intestinal wall and are then carried by the circulation to be deposited in tissues (usually muscle). There they develop into cysticerci larva, which are white and ovoid, measuring approximately 8 x 5mm. Humans become infected by ingesting inadequately cooked beef or pork with cysticerci, containing an invaginated protoscolex. The protoscolexes evaginate and pass into the small intestine where they attach themselves to the mucosa and develop into adult worms. Eggs and proglottids are passed out in feces, and are then eaten by the intermediate host, thus, perpetuating the life cycle. Morphology Ova of Taenia species are spherical, yellowish brown and measure 31 – 34m in diameter. The shell is thick and radially striated. Within the shell, the onchosphere has 3 pairs of hooklets. However, the microscopical appearance of the ova of T. saginata and T. solium are identical. The length of the adult T. saginata is 4 – 8 meters long and that of T. solium is 3 – 5metres long. The proglottids of Taenia species can be identified by the number of uterine branches; 7 – 13 for T. solium and 15 – 20 for T. saginata. If the scolex is recovered, the 4 suckers and rostellum of hooklets of T. solium will distinguish it from T. saginata, which has 4 suckers but no hooklets. Clinical Signs of Disease The presence of the adult worm rarely causes symptoms apart from slight abdominal irritation with diarrhoea, constipation or indigestion. The accidental ingestion of the embryonated ova of T. solium may result in cysticercosis in man (Fig. 21). An infection due to an adult Taenia, in man or animals, is referred to as taeniasis. T. saginata (the “beef” tapeworm) does not cause human cysticercosis. When the embryonated eggs are ingested, the embryos hatch out, migrate through the intestinal wall and are carried around the body in the circulation and deposited in various tissues (Fig. 22). Muscle and subcutaneous tissues are usually infected, but cysticerci can infect most organs and tissues. Human cysticercosis is usually asymptomatic unless the infection is particularly heavy or cysticerci are formed in some vital area e.g. the brain, resulting in neurological sequelae. Table 7 Comparison of T.saginata and T.solium Characteristic Intermediate host Site of development Scolex: adult worm Scolex: cysticercus Proglottis: uterine branches Passing of proglottids Ovary Vagina: sphincter muscle Taenia saginata Cattle, reindeer Muscle, viscera No hooks No rostellum 23 (14 – 32) * Single, spontaneous 2 lobes Present Taenia solium Pig, wild boar Brain, skin, muscle Hooks Rostellum & hooks 8 (7 –11) * In groups, passively 3 lobes Absent * No universal agreement to the number of uterine branches in these 2 species. As a rough guide, specimens with more than 16 branches are likely to be those of T. saginata and those with less than 10 branches are ikely to be of T. solium. Laboratory diagnosis Diagnosis of intestinal taeniasis can be made by recovery of the characteristic ova in stool. However, the ova of T. solium and T. saginata are identical and diagnosis is made by the recovery of the segments or scolex. The diagnosis of cysticercosis depends upon serology. MRI scans may reveal the presence of lesions in the brain. Calcified cysticerci are less often seen in the brain: in about one-third of cases, 10 years or more after infection. Occasionally, the diagnosis is made histologically on surgical specimens. Calcification in muscles usually appears 3 – 5 years after initial infection, and are most typically seen as spindle-shaped calcifications, most numerous in the thighs. Western Blots Various Immunodiagnostic tests appear to give good results on serum or CSF. Diagnosis using an immunodiagnostic test can be made using in vitro qualitative assay for the detection of IgG antibodies in serum reactive with T. solium antigens present on the membrane. Infected individuals develop a predominately IgG response to the parasite. ELISA has been used as a screening test, but low sensitivity and frequent artifactual crossreactions, or crossreactions with antibodies from other parasitic infections, limit its usefulness as a confirmatory diagnostic test. The Western Blot assay (U.S Patent No. 5,354,660) developed by Tsang et al, at the U.S. Centers for Disease Control has been shown to provide a reliable method for evaluation of sera from patients with clinically diagnosed active cysticercosis. Field studies support a sensitivity of 98% and specificity of 100% for this assay. This assay is known as the QualiCode Cysticercosis Kit, the principle behind the test is that it is a qualitative membrane-based immunoassay manufactured from T. solium proteins. The T. solium proteins are fractionated according to molecular weight by electrophoresis on a polyacrylamide slab gel (PAGE) in the presence of sodium dodecyl sulfate (SDS). The separated T. solium proteins are then transferred via electrophoretic blotting from the gel to a nitrocellulose membrane. This antigenbearing membrane has been cut into strips for testing of individual samples. Sera are tested at 100X dilution. Intersep is now the leading suppliers of Qualicode Western Blot Kits. Intersep now supply the kits for cysticercosis, hydatid disease, babesiosis, human granulocytic ehrlichiosis, canine and human lymes disease. The Qualicode line of immunoassay kits is used to detect the presence of antibody (either IgG or IgM) to a specific infectious disease agent. These qualitative assays have the sensitivity and specificity of confirmatory tests. The kits are designed for batch testing any number of samples from 1 – 24. Interpretation is simplified by including Reference Strips, Positive and Negative Controls and a Record sheet with every kit. All reagents to perform the assay are provided along with three 8-channel incubation trays. During the procedure, the strips containing the T. solium proteins are incubated with serum specimens and washed to remove unbound antibodies. Visualisation of human immunoglobulins specifically bound to T. solium proteins is performed by sequential reaction with goat anti-human immunoglobulin-alkaline phosphatase conjugate and BCIP/NBT substrate. Bands corresponding to the positions of the resoled T. solium proteins will be visualised on the strip, indicating the presence in the serum sample of IgG antibodies direct against Taenia antigens. Band positions are compared to those on a reference strip developed using the cysticersosis positive control. Hymenolepis nana Introduction Hymenolepis nana, the dwarf tapeworm, is the smallest tapeworm to infect humans. This cestode belongs to a large family known as Hymenolepididae. The diagnostic features of this family are: scolex armed with one circlet of five hooks; 1 – 3 large testes and sacciform uterus. In addition to the H.nana, three other species, H. diminuta, H. microstoma and H. citelli have been used extensively for studies on cestodes. Hymenolepis nana has a cosmopolitan distribution and is thought to be the most common tapeworm throughout the world. The infection is more frequently seen in children although adults are also infected, causing hymenolepiasis. Life cycle The life cycle of H. nana does not require an intermediate host; complete development occurs within the villi of a single host, resulting in a ‘direct’ life cycle. Though it can also utilise an insect as an intermediate host. The eggs that are released from mature proglottids in the upper ileum are usually passed out in feces. If swallowed by another human they develop into hexacanth oncospheres and burrow into the villi of the small intestine. This is where they develop into tailless cysticercoids and then migrate towards the ileum and attach thear for proglottids formation. The eggs, which are ingested by insects, such as fleas, beetles or cockroaches hatch to form tailed cysticercoids, which remain unmodified as long as they are inside the insect. If they are accidentally swallowed by a human they pass down to the ileum and establish themselves. (Peters & Gilles, 1995) Morphology The egg containing the oncosphere bears three pairs of hooklets and is surrounded by a membrane. This membrane has 2 polar thickenings from which arise threadlike filaments extending into the space between the membrane and the colourless hyaline shell, unlike those of H. diminuta, which do not possess any filaments. The adult tapeworm is normally 2.5 – 4cm long. The scolex is knob like in shape, has a rostellum with hooklets and 4 suckers. The segments are wider than they are long. Ova are spherical or ovoid measuring 30 – 47m in diameter. This is what distinguishes it morphologically from H. diminuta. Clinical Signs of Disease Infections due to H. nana may cause no symptoms even with heavy worm burdens. However, symptoms of restlessness, irritability, anorexia, abdominal pain and diarrhoea have been reported. Heavy worm burdens may be caused by autoinfection, which can be a problem in the immunocompromised. Laboratory Diagnosis Intersep has a comprehensive range of stains, fixatives and reagents for parasitology use. Preservation of parasites in faecal samples is not only important for maintaining parasite structure during transportation but also as a means of preserving parasites for future quality control and training purposes. Intersep can supply you with the most important fixatives and reagents that are used in clinical laboratories. Accompanying these fixatives, there is a range of permanent and temporary stains available to suit your needs. All stains, reagents and fixatives are supplied prediluted ready for use. Diagnosis is based on recovery and identification of the characteristic ova in a formol-ether concentrate of feces. Adult worms and proglottids are rarely seen in stool samples. Diphyllobothrium latum Introduction Members of this order, commonly known as pseudophyllids, are chiefly parasites of fish-eating mammals, birds and fish. They typically are found with a scolex which is characterised by two shallow elongated bothria situated with one dorsally and one ventrally. The proglottids are flattened dorsoventrally. Diphyllobothrium latum is an intestinal tapeworm, known as the human ‘broad’ tapeworm. It is the largest tapeworm found in man. The term ‘broad’ relates to the fact that the proglottids are generally wider than longer. It is an important human parasite. The adult worms of two other species of the genus, D. dendriticum and D. ditremum are chiefly parasite of fish-eating birds and mammals. The tapeworm, D. latum has a wide distribution, occurring especially in countries bordering on the Baltic Sea (Finland, Sweden etc.): and also in Russia, Switzerland and North America. It is in these countries where the populations are known to eat uncooked or partly cooked (i.e. smoked) fish. Apart from man they are found in many other hosts, especially the dog, cat and pig. This is due to the host countries allowing the domestic animals access to the offal from the infected fish. Life cycle and transmission The life cycle of this tapeworm requires two intermediate hosts (Fig. 23). The eggs are passed out in human faeces, once in water they hatch out into small ciliates coracidium larvae, which swim until ingested by Copepods. It is in these intermediate hosts that growth and development of the 1 st larval stage are completed (they are now known as procercoids). When these crustaceans (fresh water) are eaten by fish, the procercoid larvae continue to develop in the flesh of fish and become known as plerocercoid larvae. It is this stage of the larvae, which develops in man when they eat undercooked fish and they grow into adult worms in the small intestine. Morphology The egg is usually ovoid and has a small knob at the opercular end and is yellowish-brown in colour with a smooth shell, of moderate thickness. They measure 58 – 75m by 40 – 50m in size. Adult worms can reach up to a length of 10 metres or more and may contain up to 3,000 proglottids. The scolex is spatulate with no rostellum or hooklets. It has 2 shallow grooves or bothria, which are unlike the typical 4 suckers seen on the Taenia species. The proglottids measure 3mm long and 11mm wide and have a rosette shaped central uterus. Clinical Signs of Disease The infection caused by D. latum is due to the ingestion of raw, poorly cooked or pickled fresh water fish. The symptoms associated with D. latum infection may be absent or minimal with eosinophilia. There may be occasional intestinal obstruction, diarrhoea, and abdominal pain. The most serious symptom is the onset of pernicious anaemia. This is due to vitamin B12 deficiency, caused by excessive absorption of the vitamin by the adult worm and the absorption of cobalamins from the host intestine (occurring only in a small percentage of people). Laboratory diagnosis Laboratory diagnosis depends on the recovery of characteristic eggs from a formol ether concentrate of feces. Proglottids may also be seen in fecal samples usually in a chain of segments from a few centimeters to about 0.5 meters in length. TOPIC: NEMATHELMINTHS, NEMATODA The Nematodes Nematodes (or “round worms”) are non-segmented helminths known as make up a large assemblage of relatively simple structured organisms. They possess bilateral symmetry and a complete digestive tract with oral and anal openings; they taper to a relative point at both ends. They are also found to have separate sexes, with the male being smaller than the female, ranging in size from a few millimeters to over a meter in length. Their cylindrical non-segmented bodies allow them to be easily distinguishable from other helminths. Nematode infections have a wide spread distribution being found in both Temperate and Tropical climates. They can be found in fresh water, in the sea and the soil, successfully invading both animals and plants. The nematodes found in man invade the body fluids such as the blood or lymph channels and also the intestine. The ones that successfully invade the intestine are generally larger but, the nematodes, which invade the tissues, can grow to relatively enormous lengths. Once hatched in the intestine they undergo an incredible migration. The larvae initially burrow into the mucosa, penetrate blood vessels and appear as second stage larvae in the liver within six hours post-infection. Here they remain for several days and develop into third stage larvae, L3. These larvae then migrate to the heart and are carried to the lungs via the pulmonary arteries, arriving within 4 to 7 days. From there they break out of the capillaries into the alveoli and finally work their way up the trachea to the pharynx and reach the small intestine on the 8th or 10th day postinfection. Within the intestine, the larvae begin their third moult and become fourth stage larvae by the tenth day. The pre-patent period of A. suum in pigs (40 – 53 days) is less than that of A. lumbricoides (54 – 61 days) in humans. Two to three months after ingestion of the eggs, the females lay eggs in the intestine. The fertilised female can lay about 200,000 eggs per day. Eggs require oxygen and moisture to embryonate and the worm is often found associated with Trichuris trichiura (see the Trichuris trichiura section). Ascaris lumbricoides Morphology Ascaris lumbricoides is the largest of the intestinal nematodes found in man. The male measures 15cm with a diameter of 3 – 4mm and has a curled tail with protruding spicules. The female is 20 –35cm long with a diameter of 5mm with a straight pointed posterior end. The mouth has one dorsal and 2 ventral lips. Both are creamy white and the cuticle has fine circular striations. The ova can be unfertilised, fertilised or decorticated and can show considerable variation in shape and size. They measure 85 – 95m by 43 – 47m. The fertilised ova are easily recognised, oval in shape with a thick wall showing an irregular bumpy surface. They measure 45 – 75m by 35 – 50m. The outer covering has an albuminoid coat, stained golden brown by bile. The outer wall lies directly on top of a thick smooth shell, which is not easily distinguishable. Some have lost their albuminoid wall. The unfertilised ova are longer and narrower than the fertile ova, measuring 75 – 85m by 35 – 50m. The shell layers of the egg provide a very resistant structure, which can withstand many chemicals, which make them ideal parasites of the intestine (Fig. 24). Clinical Signs ofDisease Small burdens of worms in the intestine may cause no symptoms. The patient may have symptoms of pneumonitis with cough and low-grade fever during the migration of the larvae through the liver and lungs. This can be accompanied by wheezing, coughing and eosinophilia. In heavy worm burdens the adult worms actively migrate in the intestine resulting in intestinal blockage, vomiting and abdominal pain but infections may also be asymptomatic. The worms can penetrate through the wall of the intestine, or into the appendix, travel up the common bile duct, which may become blocked or they may then enter the gallbladder or liver. A heavy worm burden in children may lead to severe nutritional impairment and retardation in growth. Laboratory diagnosis The adults of A. lumbricoides may be expelled through the anus, mouth or nose. It is important to distinguish the adult worms from earthworms, which are segmented and are often collected as a contaminant from toilets. The microscopic examination of stool deposits after concentration reveals the characteristic bile stained ova. Eggs may be difficult to identify if an excess of iodine is added to the wet preparation as they retain the stain thus resembling debris. Ova may also become decorticated. In most symptomatic cases identification is easy due to the vast number of eggs, which can be found within a few seconds of starting to scan the slide. Hookworm species Introduction Hookworms infective to man comprise of 2 species, Necator americanus and Ancylostoma duodenale. They are classified as one of the most destructive of human parasitic helminths. There is no intermediate host, with man being the only definitive host. It is estimated that there are some 900 million cases of infection world-wide (Crompton, 1989). The infection is serious where the worms insidiously undermine the health of their hosts. They occur in areas where sanitary and environmental conditions favour the development of the eggs and larval infections (warm, damp soil). The geographic distributions of the two species are remarkably divided into: Necator americanus, which predominately is a New World hookworm, where it was introduced from Africa to the Western Hemisphere. It can also be found in the Far East, Asia, Africa, South America and Oceania; Ancylostoma duodenale is an Old World hookworm; it is the only species of Europe and areas bordering on the Mediterranean. It can also be found in the Middle East, North China, Africa, Asia and South America. Life cycle The adult worms live in the small intestine, attached firmly to the mucous membrane of the gut lining, and feed on blood and tissue (Fig. 25). The adult females deposit their eggs in the gut (they can produce up to 20,000 eggs per day); the eggs are then passed out in feces. The rhabditiform larvae hatch in warm, damp soil (light sandy loam), feeding on bacteria. After about one week during which they have gone through 2 moults, they become infective and climb into a suitable position waiting for a suitable host to pass by. The larvae enter the host by penetrating unbroken skin (it is now recognised that A. duodenale can successfully enter man by oral ingestion, this may be more important for this species than skin penetration). The larvae then enter blood vessels and are carried to the heart, lungs and trachea. They are then swallowed and develop into adult worms in the small intestine. Larvae that are initially swallowed may not show this migration. Larvae live for an average of 3 – 6 weeks in the tropics (A. Duodenale can live at lower temperatures than N. americanus can, and so is found in more temperate climates). Morphology Both species have similar general morphology and measure approximately, females 10 – 13mm and males 8 – 11mm. The general morphology of the two species resembles those of Nippostrongylus brasiliensis, the rat hookworm, but they are approximately twice the size of the rat hookworm (species not discussed here). The male species has a posterior copulate bursa, which is absent from the female. The females though possess a vulva opening, which is found almost one third of the body length from the posterior end, they also have two ovaries. Most of the female body is occupied with eggs. The mouth (or buccal cavity) of the two species show a conspicuous pair of chitinous plates on the dorsal surface. Ancylostoma duodenale buccal cavity bears 2 hook like teeth on the top and 2 triangular cutting plates on the bottom. While the mouth of N. americanus has 4 cutting plates, 2 on the ventral and 2 on the dorsal surfaces. The head is curved in both species but Necator adults it is finer but more pronounced forming a definite “hook” at the anterior end. The buccal cavity is used to attach the worms securely to the mucosa of the small intestine. With the teeth and cutting plates used to pierce the mucosa. The bursa (the characteristic external feature which forms an umbrella-like extension surrounding the cloaca) of both male species is well developed. Necator adults are distinguished from Ancylostoma by the split dorsal rays and the close arrangement of the lateral rays. The ova are oval and transparent with a smooth thin shell and measure 56 – 75m by 36 – 40m. They are usually passed in the 4 – 8 cell stage in faeces and may be embryonated. The ova of both species of hookworm are similar. Clinical Signs of Disease Larval penetration of the skin may lead to pruritis, often termed as “ground itch” at the site of penetration. Respiratory symptoms may arise during the larval migration. The adult worm in the intestine may cause intestinal necrosis and blood loss as a result of the attachment of the adult to the intestinal mucosa. Patients with acute infections may experience nausea, vomiting, abdominal pain, diarrhoea and eosinophilia. Chronic infections may lead to iron deficiency and anaemia resulting from the excessive loss of iron. Heavy worm burden in children may have serious consequences including death. Cutaneous larva migrans If man comes in contact with hookworm larva of the dog (or cat), A. braziliense or A. caninum, penetration of the skin may take place. The larvae are unable to complete the migration to the small intestine and become trapped. Trapped larvae may survive for weeks or even months, migrating through the subcutaneous tissues. Trapped larvae have been known to produce severe reaction, forming tunnels through the tissues, causing intense itchy skin eruption, producing a red track under the skin, which demonstrates accurately the wanderings of the larvae. Often intense pruritis and scratching may lead to secondary bacterial invasion, known as “creeping eruption” or “cutaneous larval migrans”. First-stage rhabditoid larvae that hatch from eggs are 250-300 μm long by 17 μm. They have a long buccal canal and their genital primordium is small and difficult to see. Infective, third-stage, filariform larvae are 500—600 μm long. These have a pointed tail and a ratio of esophageal to intestinal length of 1:4. The sheath about the larvae is conspicuously striated. Laboratory Diagnosis Adults of hookworm species may be passed out spontaneously in feces. The microscopic examination of stool deposits after concentration reveals the characteristic ova. Diagnostic Problems Eggs of this species are indistinguishable from those of Ancylostoma duodenale. If these eggs hatch in feces because of a delay in fecal examination, the first-stage larvae must be differentiated from those of Strongyloides stercoralis, which normally are passed in feces. Whereas hookworm first-stage larvae have a long buccal canal and an inconspicuous genital primordium, the larvae of Strongyloides have a short buccal canal and a prominent genital primordium. Stool specimens must not be refrigerated before attempting to culture larval stages, as Necator is especially sensitive to cold. Comments Because hookworm species cannot be differentiated on the basis of their eggs, it is necessary to culture larvae or to recover adult worms for morphologic study to make a specific diagnosis. Trichuris trichiura Introduction Trichuris trichiura, more commonly known as the “whip worm”, due to the whip-like form of the body. They have a cosmopolitan distribution, though, it is more commonly seen in tropical climates and in areas where sanitation is poor. They seem to occur in areas particularly where Ascaris and hookworm are found due to the eggs requiring the same conditions to allow for embryonation both species can be found in human together. There are several species within this genus each infecting specific hosts, but only T. trichiura infects man, causing human trichuriasis. It is a parasite that infects much more people than is generally appreciated, up to 800 million people throughout the tropics and temperate regions. Life cycle Eggs require a warm, moist environment with plenty of oxygen to ensure embryonation, but once they have embryonated they are extremely resistant to environmental conditions. Adult worms are found in the caecum and upper part of the colon of man. In heavy infection they can be found in the colon and the terminal ileum. They attach to the mucosa by the anterior end or by embedding the anterior portion of the body in the superficial tissues, obtaining nutrition from the host tissues (Fig. 26). Once fertilised the female worms lay several thousands of eggs, which are unsegmented at the oviposition and are passed out in feces. Once they have been passed out they require an embryonation period in the soil, which may last from 2 weeks to several months, after which they become infective. When embryonated eggs are swallowed by human hosts, larvae are released into the upper duodenum. They then attach themselves to the villi lower down the small intestine or invade the intestinal walls. After a few days the juveniles migrate slowly down towards the caecum attaching themselves to the mucosa, reaching their final attachment site simultaneously. The larvae reach maturity within 3 weeks to a month after infection, during which they undergo 4 moults. There is no lung migration and the time from ingestion of infective eggs to the development of adult worms is about 3 months. Infection is achieved by swallowing soil that contains embryonated eggs. Therefore, children are most commonly seen to possess the infections, as they are more likely to swallow soil. Morphology The adult worms of T. trichuria are characterised by the enormously elongated capillary-like oesophagus (anterior end) with the anus situated in the extreme tip. The thin anterior portion of the worm is found embedded in the mucosa. There are no lips, and the vulva is at the junction of the thread-like and thickened regions of the body. The posterior end is much thicker and lies free in the lumen of the large intestine. The female measures 35 – 50mm long and the male 30 – 45 mm long. The ova are characteristically barrel shaped, bile stained with bipolar plugs. They measure 50 – 54m by 20 – 23m. Clinical Signs of Disease Most infections due to this nematode are light to moderate with minimal or no symptoms. However, a heavy worm burden may result in mechanical damage to the intestinal mucosa due to the adult worm being threaded into the epithelium of the caecum. Abdominal cramps, tenesmus, dysentery and prolapsed rectum may occur in these cases. If a prolapsed rectum is observed, many worms may be seen adhering to the mucosa of the rectum. Symptomatic infections are usually only seen in children. The majority of infections are chronic and mild, with nonspecific symptoms like diarrhoea, anaemia, growth retardation, and eosinophilia. Laboratory Diagnosis The adult worms of T. trichiura are rarely seen in feces. The microscopic examination of stool deposits after concentration reveals the characteristic barrel shaped ova. In symptomatic infections numerous numbers of eggs can be seen due to the prolific nature of the female worms, even in mild infections many eggs can be seen in the smear. Strongyloides stercoralis Introduction Strongyloides stercoralis is an intestinal nematode commonly found in warm areas, although it is known to survive in the sub-tropics (hot and humid conditions). The geographic range of Strongyloides infections tend to overlap with that of hookworm due to the eggs requiring the same environmental conditions to induce embryonation. This parasite is interesting in that it contains a free-living stage (exogenous) and a parasitic stage (endogenous) where their larvae undergo development in both stages. Life cycle The life cycle of S. stercoralis is a complex one as demonstrated in the figure below (Fig. 27). The life cycle has three phases: The parasitic adult females lay eggs while they are in the duodenum where they hatchproducing rhabditiform (non-infective) larvae. 1. The larvae can have two fates in life, one where they are passed out in feces to continue down the free-living pathor they develop into infective filariform larvae whilst travelling down thesmall intestine. 2. The larvae which, develop in the environment can also undergo different development. Some larvae undergo directdevelopment (homogonic) or indirect development (heterogonic). The non-infective first stage (rhabditiform) larvae develop into free living adults in the soil within 2 – 5 days andproduce infective third stage or filariform larvae which can penetrateexposed skin (heterogonic development). This phase is common in moist, warm tropical countries. In the non-infective rhabditiform larvae, which are excreted in feces, develop into infective filariform larvae in the soil (homogonic development). These infective larvae penetrate exposed skin. There is no development of free living adult worms and this phase is common in temperate zones. The larvae never undergo sexual maturity. Both types of larvae can become established in the host by penetrating the skin or by oral ingestion. The larvae, which infect the host by penetrating the skin, undergo a migration through the dermal tissues and into the circulation to the heart and lungs, then up the bronchi and trachea, where they are eventually swallowed and pass down into the intestine. On reaching the mucosa of the duodenum the females develop and produce eggs. Adult males are unable to attach themselves to the mucosa, therefore, for any copulation to take place they must mate in the lumen of the intestine. 3. The non-infective rhabditiform larvae develop into infective filariform larvae while passing down the small intestine. Autoinfection occurs when the larvae reinfect the host by penetrating the intestinal mucosa or the perianal or perineal skin. The larvae migrate to the lungs via the circulatory system and then return to the intestine. From initial infection to maturity usually less than 4 weeks pass. Morphology The first stage rhabditiform larvae measure approximately 250m long and 20m wide. They have a bulbed oesophagus and a short buccal cavity. In an old specimen, rhabditiform larvae of S.stercoralis must be differentiated from those of hookworm, which have a longer buccal cavity. The third stage or filariform larva is approximately 500m long and has a notched tail compared with that of hookworm, which is sheathed and has a long slender tail. Adults are slender and possess and extremely long oesophagus, which in the female extends 1/3 to1/2 of the body. The anal opening is ventral and the tail is pointed. Eggs are rarely found in the stool as they hatch in the intestine. They are oval and thin shelled, resembling those of hookworm but are smaller measuring 50 – 58m by 30 – 34m. Clinical Signs of Disease Disease associated with infections due to S.stercoralisis varied, ranging from some patients being totally asymptomatic to the hyperinfection syndrome. There are 3 areas of involvement in Strongyloides infections: skin, lungs and intestine. 1. 2. 3. Initial skin penetration of the filariform larvae usually causes very little reaction, however with repeated infections the patient may mount a hypersensitive reaction, thus, preventing the larvae from completing its life cycle. The term larva currens is used when there is a rapidly progressing urticarial track. The migration of larvae through the lungs may stimulate an immune response, which can result in a cough, wheezing and fever. Symptoms associated with intestinal strongyloidiasis may mimic a peptic ulcer due to ulceration of the intestinal mucosa. In heavy infections the intestinal mucosa may be severely damaged resulting in malabsorption. There may also be lower gastrointestinal bleeding. Eosinophilia may be high. Hyperinfection syndrome The autoinfective capability of larvae may be responsible for long-term infections, which persist for many years. The parasite and host reach an equilibrium state where neither host nor parasite suffers any adverse reactions. If this equilibrium is disturbed e.g.immunosuppression, the infection proliferates with immense numbers of larvae migrating to every tissue in the body, especially the lungs. This condition is referred to as disseminated strongyloidiasis. This results in tissue damage, pneumonitis, brain damage or respiratory failure. Laboratory diagnosis. Microscopy Laboratory diagnosis depends on finding larvae in stool, sputum or duodenal aspirates. Strongyloides larvae may be present in stool in very small numbers and culture methods maybe needed to encourage the rhabditiform larvae to develop into filariform larvae and migrate from the sample. The Enterotest or string test can be used to recover larvae from duodenal aspirates. Larvae must be distinguished from hookworm larvae especially if it is an older sample. Rhabditiform larvae are most commonly seen. A good concentration technique is essential to increase the chances of seeing larvae, though they are easily killed making diagnosis more difficult. Serology Serological tests are of value in the diagnosis of strongyloidiasis when larvae cannot be found. An enzyme-linked immunosorbent assay (ELISA) using larva antigen, is usually employed. Enterobius vermicularis Classification. Helminth. Phylum Nematoda, Nematode. Disease. Enterobiasis (pinworm infection, oxyuriasis). Geographic Distribution. Worldwide. Location in Host. Cecum, appendix, colon, and rectum. Morphology. Adult Worms Males are 2.5 mm long by 0.1-0.2 mm wide, and have a blunt posterior end and a single spicule, 100-140 μm long. Females are 8-13 mm long by 0.3—0.5 mm wide, and have a long pointed tail. In both sexes, there are cephalic inflations, and the esophagus is divided into three parts — corpus, isthmus, and bulb. Eggs Elongate, flattened on one side, with a thick, colorless shell, 50-60 μm by 2030 μm. Eggs are partially embryonated when laid. Life Cycle Females usually emerge from the anus at night and discharge their partially embryonated eggs on the perianal surface (Fig. 28). Eggs embryonate to the infective first stage within 4 to 6 hours. Infection usually is by direct transmission of eggs to mouth by hands or through fomites. Parasites develop in the lower intestinal tract, and the prepatent period is 3 to 4 weeks. Adults normally live for only a few months. Diagnosis Eggs usually are detected in cellulose tape preparations applied to the patients perianal region in the early morning prior to the patient's bathing or using the toilet. Eggs are sometimes found in fecal preparations; however, routine diagnosis by fecal examination is unreliable because eggs are not introduced into the fecal stream. Instead these eggs are discharged onto the surface of fecal material as it passes through the rectum. Not infrequently, adult females are seen around the anus or on the surface of stool specimens. Rarely, immature larval stages of pinworms, especially female worms, are found in fecal specimens. These developing larvae lack cephalic inflations for their first 2 to 3 weeks of development, but the characteristic morphology of the esophagus seen in adult worms is present in these larvae and aids in the correct diagnosis. Diagnostic Problems Because eggs are not usually found in routine fecal examinations, cellulose tape preparations are the most reliable means for detecting this infection. Comments Enterobiasis is a familial and group infection that is more prevalent in children. It is very common in daycare nurseries and institutional settings. A second species, E. gregorii, has been observed from humans; males can be distinguished from those of E. vermicularis by having a short spicule (70-80 μm long). Trichinella spiralis Introduction Trichinella spiralis was first seen by James Paget but was named and described by his Pofessor, Richard Owen. The family Trichinellidae contains only one single genus Trichinella and was originally thought only to contain the one species, Trichinella spiralis, which causes the serious and often fatal disease in man known as trichinosis (trichinelosis). It is a parasite of carnivorous animals and is especially common in rats and in swine fed on uncooked garbage and slaughter house scraps, humans become infected by eating raw pork, with sausages being the most common cause of infection. It is a cosmopolitan parasite and prevalent in many European countries with the highest interest being in China. It is now thought that there are five varieties of this species that exists world-wide: Trichinella spiralis spiralis – temperate zone – high infectivity for pigs, rats and man. Trichinella spiralis nelsoni – tropics – low infectivity for pigs and rats and high infectivity for lions, hyenas. Trichinella spiralis nativa – Arctic – low infectivity for pigs, found in polar bears, resistant to freezing. Trichinella spiralis pseudospiralis- New Zealand – low infectivity for pigs, rats and mice. Trichinella spiralis is a “domestic” parasitic nematode long recognized to cause a zoonosis transmitted to man by the ingestion of infected pork. Life cycle Infection in the definitive hosts is acquired by the hosts eating raw or undercooked flesh e.g. pork, containing encapsulated larvae. Rats are probably the most highly infected “natural” hosts and pigs become infected by eating infected pork scraps or occasionally rats, which inhabit their stalls (Fig. 29). For man sausages are a dangerous source of the parasite as a small fragment of infected pork, (after mincing), may become widely distributed among a number of sausages. Humans become infected by eating raw meat containing encysted larvae. The cyst becomes digested and releases the larvae, which invade the intestinal mucosa. They develop and mate in the second day. By the 6th day of infection, the female adults deposit motile larvae, which are carried by the intestinal lymphatics or mesenteric venules to other tissues in the body. The very active muscles, such as the diaphragm, jaws, tongue, larynx and eyes, are invaded and the larvae become encapsulated by the 21st day following infection. Calcification of the cysts occurs as early as five months, but usually begins after 6 –18 months. The cyst wall is derived from the host's muscle fiber and the larvae remain viable for many years with no further development occurring. When muscles are eaten by the definitive host, sexual maturation in the intestinal phase, as explained earlier, occurs rapidly. Morphology The adult female worm is about 2-3mm long and 90m in diameter. The male is smaller measuring 1.2mm long by 60m in diameter. The female adult worms are ovoviparous and up to 1500 larvae may be released by a single worm. Clinical Signs of Disease Symptoms during the intestinal phase may go unnoticed or may be severe. Epidemics can result in outbreaks of gastro-enteritis, 2 to 7 days after the ingestion of raw pork. Diarrhoea with or without abdominal pain may last for several weeks. Eosinophilia and fever occur in most cases. Leucocytosis is common and hyperglobulinaemia is characteristic. Myocytosis and circum orbital oedema are classical signs. There can also be central nervous system involvement. Pathogenicity The primary pathogenic effect of Trichinella comes from the destruction of the striated muscle fibres in which it encysts. There can be neurological manifestations of trichinosis and death may be ascribed to myocarditis, encephalitis or pneumonitis. Laboratory diagnosis Diagnosis of trichinosis depends on the clinical signs, such as myalgia, periorbital oedema, fever and eosinophilia in a patient with a history of eating pork or sausages. Serological tests are available but may be negative if carried out within 3 – 4 weeks after infection. Circulating antibodies to T. spiralis appear on 2 – 4 weeks after infection. Redefined diagnostic antigens for their detection are currently being developed. A simple IFAT employing fragments of larvae, as antigen is a useful diagnostic tool. Latex tests with extracted larval antigens are also proved to be valuable in the acute stage, during which high antibody titers develop. Muscle biopsy is available with the muscle being digested in pepsin, which frees the encapsulated larvae or by a simple device whereby the muscle sample is compressed between 2 glass plates to make it semi-transparent, allowing you to see any encapsulated larvae using a “trichinoscope” (a simple magnifying system). The Blood Nematodes These nematodes are known as filariae and consist of a group of nematodes, which have successfully invaded the blood stream, connective tissue, or serous cavities of vertebrates. They are long thread –like nematodes. Many of them are of medical and veterinary importance attacking man and various domestic animals being transported by various vectors, including mosquitoes. The nematodes from this order do require intermediate hosts for the completion of their life cycle. The morphology of these nematodes consists of a cylindroid pharynx with an anterior muscular portion and a posterior glandular portion; the males have spirally coiled tails. Sexually mature female worms release microfilaria, which are pre-larval stages. These are released into the bloodstream. Most species are known to be ovoviviparous and some have “sheathed” microfilaria. The filarial nematodes which parasitise in man consist of Wuchereria bancrofti, Brugia malayi, Brugia timori and Loa loa, Onchocerca volvulus, Mansonella perstans, Mansonella streptoserca and Dipetalonema streptocerca. They inhabit a range of locations within the body: lymph glands, deep connective tissue, subcutaneous tissues or mesenteries. Invasions of these tissues usually result in inflammatory reactions, which is a typical symptom of a human filarial infection. In some cases these result in fleshy deformities known as elephantiasis. It has been estimated that approximately 1 billion people in tropical and subtropical countries are exposed to the risk of filarial infections and at least 200 million are infected with filariasis. The species which are primarily responsible for these human filarial infections are: Wuchereria bancrofti, Brugia malayi and Onchocerca volvulus. Wuchereria bancrofti Introduction Wuchereria bancrofti is a nematode causing lymphatic filariasis throughout the tropics and subtropics and is transmitted by mosquitoes. There are two strains of W. bancrofti: 1. The nocturnal periodic strain which is widely distributed in endemic regions i.e. Africa, India and the Far East and also parts of China, Korea and Japan, the microfilariae are in their highest concentrations between the hours of 10pm and 2am. 2. The sub-periodic strain which is found in the Pacific region, and has a microfilaraemia all the time with the highest numbers being detected between noon and 8pm. Humans are the only known reservoir host of W. bancrofti. Infection rates in some communities in East Africa exceed 30% of adults causing revolting swellings of the legs or genital system, known as elephantiasis in man. The adult worm occurs in tightly coiled nodular masses in the major lymphatic ducts. The main vector is Culex quinquefasciatus, a mosquito that is particularly common in towns and cities, breeding in organically polluted water, resting in houses and feeding by night on their human occupants. Typical breeding sites include: storm drains blocked with domestic refuse, accumulations of domestic waste water, inadequately covered septic tanks and pit latrines. In rural areas throughout Africa Anopheles gambiae and Anopheles funestus are involved in transmission. Elsewhere other anopheline mosquito species may transmit bancroftian filariasis in rural areas, while in Papua New Guinea Mansonia may act as a vector. Life cycle Microfilariae enter the host during a blood meal when the vector, a mosquito, punctures the skin. The infective larvae enter through the wound and migrate to the peripheral lymphatics where they grow to mature male and female worms (Fig. 30). They can live there for several years. After mating, the gravid females release sheathed microfilariae into the peripheral blood where they can be detected 8 – 12 months after the initial infected bite. The mosquito acquires the infection by ingestion of the microfilaria in the blood meal. The microfilariae lose their sheath on arrival in the stomach of the mosquito due to gastric juices. The larvae migrate to the thoracic muscles and develop into infective larvae over a period of 6 – 14 days. The larvae then migrate to the mouthparts of the mosquito, which infects the host during a blood meal. The blood stages of filariae, mifcrofilariae, vary in the times when they are present in the peripheral blood, corresponding with the peak biting time of the vector. Thus, in nocturnally periodic forms the microfilaria are present in the peripheral blood circulation at night; during the day they reside in the deep tissues, particularly the lungs. Morphology The adult worms are white and threadlike. The male measures between 2.5 – 4cm whereas the female is larger, measuring between 8 – 10cm. The microfilariae are 230 – 275m in length. The tail of the microfilariae of W. bancrofti tapers to a delicate point and exhibits no terminal nuclei. The sheath the microfilariae of W. bancrofti stains with haematoxylin stain. Clinical Signs of Disease Many patients are asymptomatic. Patients may present with fever, lymphangitis and lymphadenitis. Lymphangitis commonly affects the lower extremities and there may also be genital and breast involvement. An inflammatory reaction occurs in the lymphatic vessels that harbour the adult worms. Oedema develops which may resolve after the first few of attacks. A late complication resulting in thickening and verrucous changes in the skin known as elephantiasis may occur after recurring lymphangitis. Secondary bacterial and fungal infections may occur in patients with long-standing elephantiasis. Obstruction of the genital organs may result in hydrocoele formation and scrotal lymphoedema. Obstruction of the retroperitoneal lymphatics may cause the renal lymphatics to rupture into the urinary tract producing chyluria. Some patients with filariasis do not exhibit microfilaraemia but develop tropical pulmonary eosinophilia, which is characterised by peripheral eosinophilia, wheeze and cough. High eosinophilia, high IgE level and high anti-filarial antibody titres are features of this syndrome. Laboratory diagnosis Sheath may or may not stain with Giemsa; is stained with haematoxylin stains. Discrete nuclei. Empty space between the nuclei and the body wall. No nuclei in tip of tail. Innerbody is rarely visible in Giemsa. Is not stained with haematoxylin. Cephalic space as long as it is broad. Tip of tail may be bent underneath the body. Found in blood. Brugia malayi Introduction Brugia malayi is a nematode causing lymphatic filariasis in South East Asia. There are two strains of B.malayi: 1. The nocturnal periodic strain, which is widely distributed in Asia, the microfilariae are in their highest concentrations between the hours of 10pm and 2am. 2. The sub-periodic strain which is found in Malaysia, Indonesia and the Philippines where humans exhibit a microfilaraemia all the time with the highest numbers being detected between noon and 8pm. Nocturnally periodic Brugian filariasis is primarily a rural disease, being transmitted by various Anopheles species of mosquitoes and also by Mansonia, a mosquito that usually bites during the night. Nocturnally sub-periodic B. malayi is transmitted almost exclusively by Mansonia species, often, different species than those involved in transmitting the periodic form. Mansonoa bonneae are important vectors in Malaysia, breeding in swamp forest and biting by night, although sometimes by day as well. This species like W. bancrofti also parasitises the lymph nodes and lymphatics; the adults of the two species are indistinguishable, causing Malayan filariasis. Life cycle The adult worm inhabits the lymphatics and the female produces sheathed microfilariae, which circulate in the peripheral blood. The mosquito acquires the infection by ingestion of the microfilaria in the blood meal. The microfilaria lose their sheath on arrival in the stomach of the mosquito. The larvae migrate to the thoracic muscles and develop into infective larvae over a period of 6 – 14 days. The larvae then migrate to the mouth parts of the mosquito and enter the skin of the definitive host through the puncture wound when a blood meal is taken. The infective larvae enter the peripheral lymphatics where they grow to mature male and female worms. Morphology The adult worms of B. malayi are smaller than those of W. bancrofti. The microfilariae of Brugia malayi are 170 – 230m in length and have 2 terminal nuclei that are distinctly separated from the other nuclei in the tail. The last terminal nucleus is quite small and is at the tip of the tail. The sheath stains deep purple with haematoxylin stain. Clinical Signs of Disease Clinical features of B. malayi are similar to those of W. bancrofti, however in B. malayi, unlike Wuchereria bancrofti, genital involvement, hydrocele and chyluria are rare. Many patients are asymptomatic. Patients may present fever. Lymphaginitis and lymphadenitis develop in the lower extremities. An imflammatory reaction occurs in the lymphatic vessels that harbour the adult worms. Oedema develops which may resolve after the first few attacks. However, in prolonged disease after several episodes of lymphaginitis, thickening and verrucous changes in the skin occur, known as elephantiasis. Some patients with lymphatic filariasis do not exhibit microfilaraemia. However, they do have high eosinophilia, high IgE level and high anti-filarial antibody titres. Laboratory diagnosis Kinked microfilaria. Sheath is stained deep pink with Giemsa stain. Is stained with haematoxylin stains. Nuclei crowded and fill the whole body. Empty space between nuclei and body wall. Cephalic space twice as long as it is broad. Innerbody may or may not be stained; when it is, it is prominent. Found in blood. Brugia Timori Brugia timori is found in the islands of Indonesia and exhibits a strictly nocturnal periodicity. The lifecycle and disease closely resembles that of Brugia malayi. However, the microfilariae can be distinguished from those of B. malayi in that they are about 310m in length. The sheath is satined pink with Giemsa and the nuclei at the tip of the tail are similar to those of B. malayi Loa loa Introduction Loa loa, also known as the African eye worm, is a filarial nematode endemic in the rain forests of West and Central Africa. It is transmitted by Chrysops species, also known as mango flies or horse flies, and humans are the only known reservoir. It is estimated that 2-13 million humans are infected with the larvae. Adults migrate in the subcutaneous tissues of man and monkeys, with them eventually migrating across the eyeball under the conjunctiva. Life cycle The adult worms live in the subcutaneous and deep connective tissues and the microfilariae are found in the peripheral blood, where they can be in ingested by the Crysops fly (day biting fly). The adults can live in the tissues for up to 17 years (Fig. 31). Once the microfilariae have been taken up by the Chrysops during a blood meal they develop within the fat body. They develop through to L3 within 10 – 12 days. The microfilariae, L3 re-enter the host’s blood stream when the fly takes another blood meal. They reach adult worms within 4- 6 months living in the subcutaneous and deep connective tissues. The microfilariae exhibit diurnal periodicity, the highest numbers being detected in blood between 10am and 2pm. Morphology Adult males of Loa loa are 2 – 3.5cm long and the females from 5 – 7cm. The microfilariae of Loa loa are 250 – 300m. They possess a sheath, which stains bluegrey with Delafield’s haematoxylin. The sheath is not stained with Giemsa. The tail gradually tapers to a rounded end, the densely packed nuclei extending to the tip. Clinical Signs of Disease Many patients infected with Loa loa appear to be asymptomatic and the migration of the adult worm through the subcutaneous tissues often goes unnoticed, unless passing beneath the conjunctiva of the eye. They can be seen crossing the eye, but it is a rapid process taking approximately 15 – 20 minutes. Hypereosinophilia and increased antibody levels, especially IgE are also noted. Eyeworm episodes are as equally common in man as well as women with common re-occurrences. There is an increased incidence with age. The most common pathology associated with Loa loa infections are Calabar swellings, which are inflammatory swellings resulting in a localized subcutaneous oedema. These swellings are due the host’s response to the worm or its metabolic products and can be found anywhere in the body but most commonly in the extremities. These swellings last from 1 – 3 days. They develop rapidly and last one to three days, usually accompanied by localized pain, urticaria and pruritis. There is a higher frequency of calabar swellings in women with common re-occurences. Serious complications such as cardiomyopathy, encephalopathy, nephropathy and pleural effusion have been recorded. Laboratory diagnosis Kinked and sheathed microfilaria. Sheath is not stained with Giemsa stain; it is stained with haematoxylin. Nuclei crowded extending to tip of tail; tip of tail tapers. Cephalic space as long as it is broad. Innerbody is not usually stained. Found in blood. Mansonella species Introduction Members of the genus Mansonella are filarial nematodes, which rarely cause serious disease. However, they can be found in geographical areas where Wuchereria bancrofti, Loa loa and Onchocerca volvulus also occur and therefore must be differentiated from these pathogenic microfilariae. Unlike the pathogenic blood filariae, they do not exhibit periodicity. Life cycle There is a general life cycle for the Mansonella species of filarial nematodes. The microfilaria are picked up by the vector Culicoides sp. (biting midges) during a blood meal. The larvae develop within the body of the Culicoides sp. and are reintroduced into the human host when the vector takes another blood meal. They are found in various sites around the human host body. Table 8 Comparison of the main human filarial nematodes Species Geographic distribution Pathogenicity Adults (site of infection) Lymphagitis, Lymphatics fever, elephantiasis hydrocoele, chyluria Lymphagitis, Lymphatics fever. Elephantiasis Wuchereria bancrofti Asia, Pacific, Tropical Africa, Americas Brugia malayi South and East Asia Dipetalonema perstans Microfilariae (characteristi cs Found in blood, sheathed, periodicity variable Found in blood, sheathed, nocturnally periodic or subperiodic Peritoneal & Found in pleural blood, cavity unsheathed, nocturnally subperiodic Subcutaneo Found in skin, us tissues unsheathed, nonperiodic Peritoneal Found in Vector Africa and South America No definite pathogenicity Culicoides (biting midges) Dipetalonema streptocerca Africa (Ghana and Congo) Mansonella Central and Cutaneous oedema, elephantiasis No definite Culicidae (mosquitoes) Culicidae (mosquitoes) Culicoides (biting midges) Culicoides ozzardi South America pathogenitis cavity Loa loa Tropical Africa Skin swellings, allergic reactions Subcutaneo us tissues Onchocerca volvulus Africa, Central and South America Skin nodules, occular complications (blindness) Subcutaneo us tissues blood, unsheathed, nonperiodic Found in blood, sheathed, diurnally periodic Found in skin, unsheathed, nonperiodic (biting midges) Chrysops (Tabanidae or Horse fly) Simulium (Black fly) Microfilaria worms found in tissue and skin The main species of microfilariae found in the skin and tissue are Onchocerca volvulus and Mansonella streptocerca. Microfilariae of Onchocerca volvulus and less often, Mansonella streptocerca migrate through the skin causing itching and skin texture changes and occasionally arrive in the eye where they cause blindness. Detection of these microfilariae is from skin snips or nodule biopsies. When high numbers of microfilariae are present, they can occasionally be found in the blood and urine. Onchocerca volvulus Introduction Onchocerca volvulus is mainly found in West Africa and Central and South America. Onchocerciasis, also known as river blindness, is a major public health problem, especially in West Africa; there an eradication program has been established. It is one of the world’s most distressing diseases of helminth origin, often resulting in blindness. Onchocerca volvulus is transmitted by the species Simulium or black fly whose breeding habitat is fast flowing rivers or streams, therefore, there is a patchy distribution of the disease as it is specified to where water courses are. The adult worms are found in nodules or onchodermata in superficial sites, but may invade other tissues. It is estimated that there are 18 million cases worldwide with 17.5 million being found in Africa. Nigeria is the most infected region. The rate of morbidity is high in relation to those with an infection. The life cycle is similar to W. bancrofti, except that the intermediate hosts are various species from the genus Simulium (Black flies), the most important species is Simulium damnosum (Fig. 32). The microfilariae are ingested by a Black fly during a blood meal, from where they are carried to the midgut where they penetrate the epithelium and migrate, via the haemocoele, to the indirect flight muscles. Here they undergo two moults, L1 – L3 and develop into infective L3 larvae which move to the mouth parts. Development is completed in 6 – 9 days. When the infected fly takes another blood meal the infective larvae are once again transmitted into another host (definitive host). The microfilariae are released from the mouth parts and transmitted directly into the hosts bloodstream. Moulting takes place form L3 – L4 within 2 – 5 days and the larvae then migrate widely through the body under the skin and between muscles, ligaments and tendons. The final moult to L5 occurs at 1.5 – 2.5 months after transmission. Male worms are known to mature in about 4 months later. Female worms initiate the formation of the nodules and the males may join later. The sexually mature female worms release microfilariae, which migrate out from the nodules into the skin and other tissues, most significantly into the eye. Morphology The whitish adult worm lies coiled within capsules in the fibrous tissue. The female can measure up to 50cm while the males are shorter measuring up to 5 cm. The microfilariae of O. volvulus are unsheathed and are usually found in the skin. They measure between 221 – 287m long. Clinical Signs of Disease Clinical manifestations are due to microfilarie in the epidermis. Light infections may be asymptomatic or cause pruritis. This leads to scratching which can result in infection. Lyphadenopathy may also be a feature of early infection. After months or years, onchodermatitis results in secondary stage of thickening due to intradermal oedema and pachydermis. There is a loss of elastic fibres resulting in hanging groin, hernias and elephantiasis of the scrotum. There is finally atrophy of the skin resulting in loss of elasticity. There is mottled depigmentation of the skin. Ocular lesions are related to the intensity of the microfilariae in the skin. Ocular lesions include sclerosing keratitis, secondary glaucoma and cataract, coroidoretinitis and fluffy corneal opacities. The major complication of onchocerciasis is the development of lesions in the eye, which may result in blindness or other distressing ocular diseases. Laboratory diagnosis 1. Analysis of Skin Snips Small amounts of skin are collected by using a needle to raise the skin and then to slice about 1 mg of skin to a depth of 0.5mm. Snips are collected from several sites, usually the shoulders or the buttocks and sometimes the chest and calves. The snips are placed immediately in 0.5ml normal saline in a microtitre plate and left for 4 hours to allow the microfilariae to migrate out of the tissues. After 4 hours, the samples are examined using an inversion microscope. The microfilariae should still be moving and can be identified from the table below. The microfilariae can also be collected by filtration or centrifugation and the deposit containing microfilariae can be stained with Giemsa at pH 6.8. 2. Analysis of Biopsies Biopsies of tissue nodules can be dabbed on to a slide to produce impression smears and then stained with Giemsa stain at pH 6.8 for the presence of microfilariae. Recent advances in diagnostic methods includes and ELISA-based antibody detection assay which utilizes a cocktail of recombinant antigens. The advantages of using this test are that it is highly sensitive (almost 100% in onchocerciasis foci). It is also highly specific (100%), it also uses finger prick blood. Therefore, reducing the painful procedure of gaining a skin snip. The disadvantages is that it requires advanced ELISA apparatus and reagents and cannot distinguish between past and present infections due to it detecting antibodies which stay present in the body for a long time after the infection. Another modern detection method is for Parasite DNA detection, which is based on the amplification of specific DNA sequences form microfilariae using molecular biology technology. The advantages of this technique are its exquisite sensitivity and detect active infections only. The disadvantages are that it requires specialised equipment and expensive reagents. Also it still requires a skin snip but a urine assay is a possibility for the future. Thick microfilaria. Does not have a sheath. Head often spatulate. Nuclei do not extend to the tip of the tail. Found only in the skin. Mansonella streptocerca Microfilaria of M. streptocerca were first reported in the skin of a West African patient in 1922. These microfilaria are primarily found in the skin but have been also reported in the blood. This species occurs in Ghana, Cameroon and Zaire. The adults are poorly known, and occur in the cutaneous tissue of man and chimpanzee. The microfilariae do not exhibit periodicity with the intermediate hosts being Culicoides grahamii and possibly other Culicoides species. Life cycle The life cycle is the same as that of the blood Mansonella species. Clinical Signs of Disease Infection is characterised by pruritic dermatitis and hypopigmented macules. Laboratory diagnosis Mansonella streptocerca can be diagnosed by demonstrating the microfilaria in a skin snip. Snips are collected from several sites, usually the shoulders and buttocks and sometimes the chest and calves. The snips are placed immediately in 0.5ml of 0.9% sodium chloride in a microtitre plate and left for 4 hours to allow the microfilaria to migrate out of the tissues. After 4 hours, the samples are examined using an inversion microscope. The microfilaria should still be moving and can be identified by staining with Giemsa at pH 6.8 Small, thin, microfilaria. Does not have a sheath. Nuclei extend to the end of the tail. The tail is hooked; its tip is rounded or forked. Found only in the skin. Table 9 Differential features of Onchocerca volvulus and Mansonella streptocerca Distribution Vector Adult location Microfilariae location Microfilariae size Morphology Tail nuclei Onchocerca volvulus Tropical Africa, Central and South America Simulium spp. Subcutaneous nodules Skin 280 – 330 m Broad spatulate head No sheath, pointed tail Tail free from nuclei Mansonella streptocerca West Africa Culicoides spp. Cutaneous connective tissue Skin 180 – 240 m Curled tail No sheath Nuclei extend to tail tip Dracunculus medinensis Introduction Dracunculus medinensis is a non-filarial parasite as it only has one uterus whereas filaria have two. It is usually associated with places where there is a lack of clean drinking water e.g. step wells in India, covered cisterns in Iran, and ponds in Ghana. The life cycle usually involves copepod intermediate host. They are parasitic in the connective tissue or coelom of vertebrates. The disease associated with this parasite is known as Dracunculiasis (Fig. 33). Mature female worms, which are gravid with microfilariae, migrate to the superficial layers of skin of humans, especially those regions, which are most likely to come in contact with water, such as the ankle, foot, arms and shoulders. Here the worms secrete a substance (substance is unknown), which causes a blister to rise over its anterior end where it has pierced the lower layers. The blister eventually forms into an ulcer which on contact with water, the uterus is projected out of the ulcer cavity, and a cloud of milky white secretion, containing hundred of active larvae, is released. Once out of the water again the uterus dries and shrivels preventing the release of further larvae. If the microfilariae is ingested by an appropriate species of Cyclops, they break though the soft mid-intestine wall and come to lie in the body cavity. The larvae undergo two moults and become infective in approximately 3 weeks. Humans become infected by accidentally ingesting through drinking water the infective Cyclops. Upon ingestion the larvae are activated to penetrate through the gut wall, and migrate through the tissues, moulting twice and finally becoming lodges in the viscera or subcutaneous tissues. Maturation of the worms is slow taking about 1 year to reach sexual maturity before the females are ready to migrate to the skin to release their larvae. Morphology The adult female worm measures up to 1 metre in length whereas the male measures about 2cm. Clinical Signs of Disease After ingestion of the Cyclops, there is no specific pathology associate with the mucosal penetration and larval maturation in the deep connective tissues. Erythema and tenderness can be associated with blister formation. The patient can also exhibit vomiting, diarrhoea, asthmatic attacks. Symptoms usually subside when the lesion erupts. If the worm is removed, healing usually occurs without any problems. If the worm is damaged or broken during removal, there may be intense inflammatory reaction with possible cellulitis along the worms migratory tract. This can result in arthritis and synovitis. Laboratory diagnosis The best remedy for removing the adult worm is a slow process of daily gently rolling the worm around a small stick and slowly pulling it out of the skin. With this method you must be careful not to pull apart the worm as it will recoil back into the skin and cause secondary infections. At this moment in time this parasite is being effectively controlled due to a strict control program. The program includes stopping people from drinking infected water, putting muslin over the water jars, which they use to collect the water in, thus preventing the cyclops from being collected in water. Educating the communities about the parasite and adding temphos to the water, thus, killing off any microfilariae in water. Identifying Intestinal Helminths The usual diagnostic stages for identifying medically important helminths are the eggs and larvae. Occasionally, adult worms like Ascaris and Enterobius may be seen and segments or proglottids are used for diagnosing certain tapeworms. If an egg is found the following features as described should be carefully observed in order to make a specific identification. 1. Size: The length and width are measured and are generally within a specific range. 2. Shape: Each species has its own particular shape. 3. Stage of development when passed: In some species, the eggs consist of a single cell; in some, there may be several cells; and some species are usually embryonated (i.e., they contain a larva) when passed in feces. Occasionally, if stool specimens are several hours or 1 – 2 days old, eggs may develop to more advanced stages. Ascaris eggs usually have only 1 cell when passed in feces; however, the single cell may divide and, in old specimens, eggs with 2 or 4 cells may be seen. Hookworm eggs in specimens that are several hours old may contain 16, 32 or more cells. In 12 – 24 hours, the egg may be embryonated and later still the larvae may hatch. Therefore, when observing the stage of development of helminth eggs, be sure that the stool specimen is freshly passed. If it is several hours or a day old, expect to see changes in the stage of development of some species. Ideally only fresh samples should be accepted for diagnosis. 4. Thickness of the egg shell: Some species, like Ascaris, have thick shells; others, like hookworm, have thin shells. 5. Colour: Some eggs are colourless (e.g., hookworm, Enterobius), others are yellow or brown (Ascaris, Trichuris). 6. Presence of characteristic like opercula (lids), spines, plugs, hooklets, or mammillated outer coats. TOPIC: ARTHROPODA, ARACHNOIDEA, ACARINA Phylum Arthropoda Arthropods are said to be the most successful phylum. It includes about 80% of all species of animals with over one million species known, although it's thought that over six million could exist. This phylum includes the most diverse species in terms of distribution, being found in water, on land, and in the air, and it includes the highest number of individuals within each species. They are found in a variety of environments, from mountain tops to deep oceans, and are able to survive in tremendous environmental conditions. There are several features characteristic of all arthropods, including a hardened exoskeleton, specialized segments, jointed appendages, modified mouthparts, and specialized respiratory structures such as gills, trachea, or book lungs. The sexes are usually separate. The group includes a variety of feeding forms: herbivores, carnivores, and filter feeders to name a few. Arthropods include an incredibly diverse group of taxa such as insects, crustaceans, spiders, scorpions, and centipedes. There are far more species of arthropods than species in all other phyla combined, and the number of undescribed species in the largest assemblage of arthropods, the insects, probably numbers in the tens of millions. Members of the phylum have been responsible for the most devastating plagues and famines mankind has known. Yet other species of arthropods are essential for our existence, directly or indirectly providing us with food, clothing, medicines, and protection from harmful organisms. A number of important characteristics are shared by most members of this phylum. Arthropods are bilaterally symmetrical protostomes with strongly segmented bodies. Segmentation affects both external and internal structure. Some segments are fused to form specialized body regions called tagmata; these include the head, thorax and abdomen, and the process and condition of fusion is called tagmosis. The body is covered with an exoskeleton made up primarily of protein chitin; lipids, other proteins, and calcium carbonate also play a role. Primitively, each body segment bears a pair of segmented (jointed) appendages; in all living arthropods, many of these appendages are dramatically modified or even lost. Arthropods generally grow by molting their exoskeletons in a process called ecdysis. Movement of appendages is controlled primarily by a complex muscular system, divided into smooth and striated components as in chordates. Cilia are not present. Most arthropods have a pair of compound eyes and one to several simple ("median") eyes or ocelli; either or both kinds of eyes may be reduced or absent in some groups. Arthropods are eucoelomate with the coelom formed by schizocoely, but the volume of the coelom is much reduced and usually restricted to portions of the reproductive and excretory systems. Most of the body cavity is an open "hemocoele," or space filled loosely with tissue, sinuses, and blood. The circulatory system is open and consists of the heart, arteries, and open spaces of the hemocoele. The gut is complete. Respiration takes place through the body surface, and/or by means of gills, tracheae, or book lungs. The nervous system is annelid-like, with a brain (=cerebral ganglion) and a nerve ring surrounding the pharynx that connects the brain with a pair of ventral nerve cords. These cords contain numerous ganglia. Most arthropds are dioecious and have paired reproductive organs (ovaries, testes). Fertilization is internal in most but not all groups. Most lay eggs, and development often proceeds with some form of metamorphosis. Subphylum Chelicerata Class Merostomata (horseshoe crabs, eurypterids) Class Pycnogonida (sea spiders) Class Arachnida (spiders, ticks, mites) Subphylum Crustacea Class Remipedia Class Cephalocarida Class Branchiopoda (fairy shrimp, water fleas, etc.) Class Maxillopoda (ostracods, copepods, barnacles) Class Malacostraca (isopods, amphipods, krill, crabs, shrimp, etc.) Subphylum Uniramia Class Chilopoda (centipedes) Class Diplopoda (millipedes) Class Insecta Crustacea Crustaceans are members of the phylum Arthropoda. They are primarily marine, but many also inhabit freshwater and terrestrial habitats from the deep-sea to the highest mountain lakes. More than 52,000 species of crabs, shrimps, lobsters and their close relatives have been described; that figure is twice the number of all amphibians, reptiles, birds, and mammals combined! Although the insects still rule in terms of numbers, the crustaceans are the most diverse in their forms. The largest of the crustaceans include the the giant Japanese spider crab (Macrocheira kaempferi) with its four-meter legspan, the Alaskan king crab (Paralithodes camtschatica), which can weigh more than 10 kilograms, and the giant Tasmanian crab (Pseudocarcinus gigas), which has been recorded at an impressive 14 kilograms. On the other end of the spectrum, some crustaceans never grow larger than 0.25 millimeters, even as adults. Crabs, shrimps, and lobsters are well-known crustaceans. However, barnacles, pillbugs, amphipods, copepods, krill, crayfishes, sea fleas, clam shrimps, fairy shrimps, and many others also belong to the Crustacea, an ancient group that arose in the early Cambrian nearly 600 million years ago. The Arachnida The Arachnida include the terrestrial chelicerates that everyone is familiar with, and that nearly everyone would rather not be too familiar with: spiders (Araneae), ticks and mites (Acari), and scorpions (Scorpiones). Arachnids also include a number of less familiar taxa: Opiliones (harvestmen or daddy-longlegs); Thelyphonida (whip-scorpions); Pseudoscorpiones (false scorpions); and many others. Most are predators, and some are venomous. All are terrestrial, except for some mites and spiders that have become secondarily aquatic. Scorpions Scorpions are the oldest arachnids for which fossils are known, and they were the first arachnid fossils to be found in Paleozoic strata (Fig. 34). The Silurian scorpions appear to have lived in the water, since their fossils have gills, but by the Carboniferous scorpions with such features are no longer found –- fossils from the Pennsylvanian age Mazon Creek beds have book lungs covered by protective plates, and so were probably land-dwellers. The best scorpion fossils come from the Devonian and the Oligocene; there is a severe lack of fossils known from the intervening period. These earliest scorpiones are considered to be Protoscorpions, since they possess many traits, which are plesiomorphic for scorpions. For example, in all scorpions the thick front portion on the abdomen is made up of seven segments, but the number of sternite plates, which cover this region varies among the earliest fossils, while all living species have five. All scorpions have an additional five segments after the initial seven, ending in a sharp sting. This sting contains a pair of poison glands, which can paralyze prey, usually insects or small rodents, or may deliver a painful sting to incautious persons. Most scorpion stings are merely painful, leading to swelling in the immediate region of the sting, but some scorpions of northern Africa and the American southwest can be deadly. In the US, the deadliest scorpions are to be found in Arizona, where it is a good idea to shake out shoes before putting them on in the morning! Besides their unusually long and dangerous tails, scorpions also differ from other arachnids in having large pedipalps. These are the second pair of appendages on the body, and are usually rather inconspicuous in arachnids, but in scorpions, they are large and powerful pincers, which may be used to grasp and subdue prey. Scorpions may also have more eyes than other arachnids, some species possessing as many as six pairs, though most do not have this many. They have three joints in their chelicerae, or the first pair of appendages, located next to the mouth. Most scorpions are nocturnal, hiding under rocks, in crevices, or within burrows during the day, and coming out after sunset. Because of this, and because of their painful stings, it can be dangerous to travel at night in scorpion territory without shoes, even inside homes. One unusual feature of scorpions that has helped many field biologists is the UV fluorescence of scorpion bodies. Biologists hunting for scorpions wave an ultraviolet light near the ground as they walk along, watching for an eerie greenish light to be reflected back. The UV light is absorbed by the scorpion's armor and is reflected back as visible light. Solpugids Description: The solpugids (sun spiders, wind scorpions; order Solpugida) are spiderlike and hairy, with two closely placed median eyes. Their most striking characteristic is the enormous size of their chelicerae. These projects in front of the head, and each of the pair is composed of two pieces forming a pincer that works in a vertical plane. The pedipalps are leglike, but have a specialized adhesive terminal segment. The pedipalps and the first pair of legs (that have a tactile function) are usually carried above the cephalothorax while the solpugid is standing or running. Solpugids are common in the hot desert regions of the world. Biology: Solpugids are voracious predators that feed on all types of small arthropods. While they are most active at night, it is not unusual to see them out and about during the day. Prey is captured with the sticky ends of the pedipalps and passed to the formidable chelicerae for crushing. Only liquid and very fine particles are ingested; the pulp that results after a meal is discarded. Mating takes place when the male encounters a receptive female. No spermatophore is produced by the male; instead a sperm droplet is transferred from the substrate to the female gonopore (genital opening) by means of the male's chelicerae. In one southwestern species, the male turns the female on her back, emits seminal fluid directly into the female gonopore, then tamps it in with his chelicerae. After mating, the female constructs a burrow and nest in the ground, where she lays 50-200 eggs. She remains with the eggs until they hatch. When the young solpugids hatch, they emerge from the burrow with their mother and remain together for some time while the mother captures prey to feed the entire family. Envenomation: Solpugids are commonly considered to be venomous, but poison glands have not been found to be associated with the chelicerae. It has been suggested (but not confirmed) that poisoning might result from toxins secreted through the setal (bristle) pores that can be traced along the tips of the chelicerae. Apparently authentic cases of aftereffects resulting from a solpugid bite have been recorded, but these symptoms were probably caused by bacterial infection of the wound. The solpugid has undoubtedly been maligned because of its appearance. An old wives' tale holds that animals drinking from a water trough in which a solpugid is present will die. There is no foundation for this. Treatment: If a bite results in broken skin, wash with soap and water and apply antiseptic to the wound. Precautions and Control: Don't handle solpugids. If one is found in the house, it can be brushed into a dustpan or other container and returned to the outdoors. Solpugids are usual and beneficial components of Arizona's varied ecosystems. These animals cannot be controlled, nor would it be desirable to control them. Spiders Spiders are invertebrate animals that produce silk, have eight legs and no wings. More precisely, a spider is any member of the arachnid order Araneae, an order divided into three sub-orders in newer systems: the Mygalomorphae (the primitive spiders), the Araneomorphae (the modern spiders) and the Mesothelae, which contains the Family Liphistiidae, rarely seen burrowing spiders from Asia. The study of spiders is known as arachnology, although it is often included in the more general term entomology. Many spiders hunt by building webs to trap insects. These webs are made of spider silk, a thin, strong protein strand extruded by the spider from spinnerets on the end of the abdomen. All spiders produce silk, although not all use it to spin elaborate traps. Silk can be used to aid in climbing, forming smooth walls for burrows, cocooning prey, and for many other applications. Morphology and development: Spiders, unlike insects, have only two body segments instead of three; a fused head and thorax (called a cephalothorax or prosoma) and the abdomen, supported by a hard exoskeleton composed mainly of chitin (Fig. 35). Spiders also have eight legs (insects have six), no antennae, and their eyes are single lenses rather than compound eyes. Additionally spiders have pedipalps (or just palps), which are two appendages next to their mouths that aid in manipulating food and are used by the males in mating. Respiration and circulation: Spiders have an open circulatory system, meaning they don't have true blood or veins for it to travel in. Rather, their bodies are filled with haemolymph, which is pumped through the arteries by the heart into spaces called sinuses surrounding their organs. Spiders have developed several different respiratory anatomies, based either on book lungs, a tracheal system, or both. Primitive mygalomorph spiders generally have only a pair of book lungs filled with haemolymph, where openings on the ventral surface of the abdomen allow air to enter and diffuse oxygen. Modern araneomorph spiders often have a single book lung in addition to spiracles, which deliver air into the tracheae, where oxygen is then diffused into the haemolymph. In the tracheal system oxygen interchange is much more efficient, enabling cursorial hunting (hunting involving rapid pursuit) and other advanced characteristics. Vision: Spiders usually have eight eyes in various arrangements, a fact which is used to taxonomically classifiy different species. Sometimes one pair of eyes is better developed than the rest, or there are only six pair, or no eyes at all. Several families of hunting spiders have developed well to excellent vision, such as wolf spiders and jumping spiders. However most spiders that lurk on flowers, webs and other fixed locations waiting for prey have very poor eyesight, but possess extreme sensitivity to vibrations for hunting. Defense: Some primitive spiders, like the tarantula, have a patch of urticating hairs on their abdomens for defense, which are generally absent on modern spiders. Certain other species have specialized defense tactics. For example, the Golden Wheeling spider of the desert escapes Tarantula Wasps (a species of wasp that lays it's eggs in a paralyzed spider so the larvae have enough food when they hatch) by flipping onto its side and cartwheeling away. Life cycle: The spider life cycle progresses through three stages: the embryonic, the larval, and the nympho-imaginal. Between the time an egg is fertilized and the spider begins to take the shape of a spider it is referred to as the embryonic stage (Foelix, 1996). As the spider begins to look more like a spider it enters the larval stage (Foelix, 1996). It enters the larval stage as a prelarva and, through subsequent molts; it reaches its larval form, a spider-looking, non selfsufficient animal feeding off its yolk supply (Foelix, 1996). After a few more molts, also called instars, body structures become differentiated; all organ systems are complete and the animal begins to hunt on its own; it has reached the nymphoimaginal stage (Foelix, 1996). This stage is differentiated by two sub-stages: the nymph, or juvenile stage and the imago, or adult stage (Foelix, 1996). A spider does not transition from the nymph to the imago until it has become sexually mature (Foelix, 1996). Once a spider has reached the imago stage, it will remain there until its death. Many spiders may live only about a year, but a number will live two years or more, overwintering in sheltered areas (the annual influx of 'outdoor' spiders into houses in the fall is due to this search for a nice warm place to spend the winter). Reproduction: Spiders reproduce by eggs laid in silk bundles called egg sacs. Spiders often use mating rituals (especially in the visually advanced jumping spiders) to allow the male to approach close enough to inseminate the female without triggering a predatory response. Assuming that the approach signals are exchanged correctly, the male spider must make a timely departure after mating to escape before the female's normal predatory instincts come back into operation. Unusually, sperm transmission is an indirect process. When a male is ready to mate, he will spin a web pad onto which the contents of the abdominal reproductive organs are discharged. He then dips his palps (also known as “palpi”), the small, leg-like appendages on the front of his cephalothorax, into the sperm, absorbing it. Mature male spiders characteristically have swollen bulbs on the end of their palps for this purpose, and this is a useful way to identify the sex of a spider in the field. With his palps thus “charged” he then goes off in search of a female. The act of copulation occurs when the male inserts one or both palps into the female's genital opening, known as the epigyne. He transfers his sperm into the female by contracting his palps. Very unusual behaviour is seen in spiders of the genus Tidarren, as the male amputates one of its palps before maturation and enters its adult life with one palp only. The palpi constitute 20% of its body mass, and since this weight greatly impedes its movement, the spider detaches one of the two to gain mobility. In the Yemeni species Tidarren argo, the remaining palp is then torn off by the female. The separated palp remains attached to the female's epigynum for about four hours and apparently continues to function independently. In the meantime the female feeds on the palpless male. (Journal of Zoology (2001). Ecology: Spiders have a great range of variation and lifestyle, although all are predatory. While spiders are generalist predators, in reality their different methods of prey capture often limits the type of prey taken. Thus web-building spiders rarely capture caterpillars and crab spiders that ambush prey in flowers capture more bees, butterflies and some flies than other insects. Groups of families that tend to take certain types of prey because of their prey capture methods are often called guilds. A few spiders are more specialized in their prey capture. Dysdera captures and eats sowbugs, pillbugs and beetles, while pirate spiders eat only other spiders. Bolas spiders in the family Araneidae use sex pheromone analogs to capture only the males of certain moth species. Despite their generally broad prey ranges spiders are one of the most important links in the regulation of the populations of insects. Every day on a meadow they devour over 10 g/m² of insects and other arthropods. Mites and ticks The mites and ticks, order Acarina or Acari, belong to the Arachnida and are among the most diverse and successful of all the invertebrate groups, although some way behind the insects (Fig. 36). They have exploited an incredible array of habitats and because of their small size (some are microscopic) most go totally unnoticed. Many live freely in the soil or water, but there is also a vast array of species that live as parasites on plants or animals. Some of the plant pests include the so-called Spider mites (family Tetranychidae) and the Gall mites (family Eriophyidae). Among the species that attack animals there are members of the Sarcoptic Mange mites (family Sarcoptidae), which burrow under the skin. Perhaps the most well known, though, is the house dust mite (family Pyroglyphidae). Insects may also have parasitic mites. Examples are Varroa destructor, which attaches to the body of the honeybee and Acarapis woodi, which lives in the tracheae of honeybees. The scientific discipline devoted to the study of ticks and mites is called Acarology. Sarcoptes scabiei Etiology Caused by the mite Sarcoptes scabiei, variety hominis, it produces intense, itchy skin rashes when the impregnated female tunnels into the stratum corneum of the skin and deposits eggs in the burrow (Fig. 37). The larvae, which hatch in 3-10 days, move about on the skin, molt into a "nymphal" stage, and then mature into adult mites. The adult mites live 3-4 weeks in the host's skin. The motion of the mite in and on the skin produces an intense itch, which may resemble an allergic reaction in appearance. The presence of the eggs produces a massive allergic response, which, in turn, produces more itching. Scabies is transmitted readily, often throughout an entire household, by prolonged skin-to-skin contact with an infected person (e.g. bed partners), and thus is sometimes classified as a sexually transmitted disease. Spread by clothing, bedding or towels is a less significant risk, though possible. Signs, Symptoms, and Diagnosis: A delayed hypersensitivity (allergic) response resulting in a papular eruption (red, elevated area on skin) often occurs 30-40 days after there may be hundreds of papules, less than 10 burrows are typically found. The burrow appears as a fine, wavy and slightly scaly line a few millimeters to one centimeter long. A tiny mite (0.3 to 0.4 mm) may sometimes be seen at the end of the burrow. Most burrows occur in the webs of fingers, flexing surfaces of the wrists, around elbows and armpits, the areolae of the breasts in females and on genitals of males, along the belt line, and on the lower buttocks. The face usually does not become involved in adults. The rash may become secondarily infected; scratching the rash may break the skin and make secondary infection more likely. In persons with severely reduced immunity, such as those with HIV infection, or people being treated with immunosuppressive drugs like steroids, a widespread rash with thick scaling may result. This variety of scabies is called Norwegian scabies. Scabies is frequently misdiagnosed as intense pruritis (itching of healthy skin) before papular eruptions form. Upon initial pruritus the burrows appear as small, barely noticeable bumps on the hands and may be slightly shiny and dark in color rather than red. Initially the itching may not exactly correlate to the location of these bumps. As the infestation progresses, these bumps become more red in color. Generally diagnosis is made by finding burrows, which often may be difficult because they are scarce, because they are obscured by scratch marks, or by secondary dermatitis (unrelated skin irritation). If burrows are not found in the primary areas known to be affected, the entire skin surface of the body should be examined. The suspicious area can be rubbed with ink from a fountain pen or alternately a topical tetracycline solution, which will glow under a special light. The surface is then wiped off with an alcohol pad; if the person is infected with scabies, the characteristic zigzag or S pattern of the burrow across the skin will appear. When a suspected burrow is found, diagnosis may be confirmed by microscopy of surface scrapings, which are placed on a slide in glycerol, mineral oil or immersion in oil and covered with a coverslip. Avoiding potassium hydroxide is necessary because it may dissolve fecal pellets. Positive diagnosis is made when the mite, ova, or fecal pellets are found. Treatment Topical (surface) medications are often effective and must be applied thoroughly to all skin from the face down, especially to areas known to be primarily affected (skin folds, etc.). Medication should remain on for more than 12 hours, and preferably 24, and then washed off. Although the mites may be rapidly killed, improvement is sometimes slow and residual inflammation may take some time to finally subside. The topical medication of choice is 5% Permethrin because it is safe for all age groups. Lindane (hexachlorocyclohexane) creams or lotions are considered historical treatments, and should be avoided because they have been shown to have neurotoxic effects in children and infants. Similarly, 5–10% sulfur ointments are considered historical. A single dose of Ivermectin (dosing: 200 µg/kg) has been reported to cure, but is an off-label use, and thus considered experimental. Additional topical treatments include 10% crotamiton (except to eyes, nose, mouth), 25% benzyl benzoate cream or lotion; permethrins offer a simpler, one-application treatment which may be applied with in a 5% cream that remains on overnight or for 8-14 hours. A person can be reinfected with scabies. Without a host, scabies mites survive for a few days. Therefore it is recommended, after treatment, to wash all material (such as clothes and bedding) that has been in prolonged contact with the infected in the last four days. To prevent reinfestation, all social contacts and members of the family, even if not infested, should be treated similarly and most importantly at the same time. Approximately 300 million cases of infestation with scabies occur worldwide annually. Scabies also occurs in dogs; see Mange. Note: Although the mange mites are not able to complete their life cycle on humans, they will cause quite a bit of itching before they finally die. The demodex mite The demodex mite is a tiny parasitic mite, which lives around human hair follicles, particularly those of the eyelashes and eyebrows (Demodex folliculorum hominis) or in sebaceous glands connected to hair follicles (Demodex brevis). Measuring between 0.1mm and 0.4mm, each mite has eight segmented legs for locomotion, a long, scale-covered body for anchoring itself in the hair follicle, and pin-like mouth-parts for eating skin-cells and oils which accumulate in the hair follicles. Interestingly, the mite's digestive system is so efficient and results in so little waste that there is no excretory orifice! With a life cycle lasting around two weeks, the mites are transferred between hosts through contact of hair, eyebrows and of the sebaceous glands on the nose. A surprising fact is that an estimated 96-98% of all people carry such mites – with up to 25 in each follicle, each person can have a potentially huge population of mites. In the vast majority of cases, the mites go unobserved, without any adverse symptoms, but in certain cases (usually related to a suppressed immune system, caused by stress or illness) mite populations can dramatically increase, resulting in a condition known as demodicosis, characterised by itching, inflammation and other skin disorders. It is quite easy to look for your own demodex mites, by carefully removing an eyelash or eyebrow hair and placing it under a microscope. A related species of demodex mite, (Demodex canis), lives on the domestic dog. While, like with humans, most dogs live with their mites without harm, a minority does not have immune systems capable of completely controlling the mites, leading to a potentially dangerous infestation called demodectic mange. While direct treatment for severe cases is possible using a drug known as Mitaban, which is applied to the animal's skin, improved nutrition and checking for other, immune-system suppressing diseases are also recommended. Demodex mites are also known to cause acne in most teenagers going through puberty. Because of the excessive sebum production, the mites become more active and this in turn causes acne. When a large amount of demodex mites are present in a hair follicle, it can cause the area to become infected and in return leaving pimples or small bumps on the face. Ixodid Ixodid or hard ticks are ticks of the family Ixodidae. Hard ticks are distinguished from Argasidae or soft ticks by their chitinous external shell. Hard ticks may remain attached to the skin of a host for long periods of time. Most tickborne diseases are carried by hard ticks, although there are some exceptions, such as relapsing fever. Tick is the common name for the small wingless arachnids that, along with mites, constitute the order Acarina. Ticks are external parasites, living by hematophagy on the blood of mammals, birds, and occasionally reptiles and amphibians. Ticks are important vectors of a number of human and animal diseases (Fig. 38). Characteristics: The major families of tick include the Ixodidae or hard ticks, which have thick outer shells made of chitin, and Argasidae or soft ticks, which have a membraneous outer surface. Soft ticks typically live in crevices and emerge briefly to feed, while hard ticks will attach themselves to the skin of a host for long periods of time. Most reside in the Northwestern US. Tick bites look like mosquito bites, but can also sometimes bruise or resemble a bullseye. Ticks as disease vectors Hard ticks can transmit human diseases such as relapsing fever, Lyme disease, Rocky Mountain spotted fever, tularemia, equine encephalitis and several forms of ehrlichiosis. Additionally, they are responsible for transmitting livestock diseases, including babesiosis and anaplasmosis. Diseases such as HIV/AIDS and malaria can be transmitted by soft ticks. Generally, tick-borne diseases correspond to a specific tick-host combination, and are limited in their geographical extent. Location: Ticks are often found in high grass, where they will rest themselves at the tip of a blade so as to attach themselves to a passing animal or human. It is a common misconception that the tick can jump from the plant onto the host. Physical contact is the only method of transportation for ticks. They will generally drop off of the animal when full, but this may take several days. Ticks contain a structure in their mouth area that allows them to anchor themselves firmly in place while sucking blood. Pulling a tick out forcefully may squeeze contents of the tick back into the bite and often leaves the mouthpiece behind, which may result in infection. Methods for removing a tick without leaving its mouthpiece inside the skin include anesthetizing the tick with a substance such as ether. According to the Rhode Island Department of Health, roughly 70% of people who develop Lyme disease catch it from ticks in their own yard. Some ways to reduce the risk of this happening include: Eliminating salt licks, birdbaths, and other features that attract wildlife; Keeping the grass short and free of clippings, leaves, and other debris that shelter ticks; Building a fence to keep out deer, since deer can carry hundreds of ticks; Creating a 3-foot wide, 3-inch deep gravel border between the yard and any adjacent wooded areas. Life cycle: Deer (black-legged) tick. The deer (or black-legged) tick, and the related western black-legged tick, are the primary known transmitters of Lyme disease in the United States. Both are hard-bodied ticks with a two-year life cycle. Like all species of ticks, deer ticks and their relatives require a blood meal to progress to each successive stage in their life cycles. The life cycle of the deer tick comprises three growth stages: the larva, nymph and adult. In both the northeastern and mid-western US, where Lyme disease has become prevalent, it takes about two years for the tick to hatch from the egg, go through all three stages, reproduce, and then die. A detailed description of this life cycle and the seasonal timing of peak activity, as they occur in these regions, is provided below. The best way to get rid of a tick is to rub it with rubbing alcohol or hold a flame to it. Larva: Eggs laid by an adult female deer tick in the spring hatch into larvae later in the summer. These larvae reach their peak activity in August. No bigger than a newsprinted period, a larva will wait on the ground until a small mammal or bird brushes up against it. The larva then attaches itself to its host, begins feeding, and engorges with blood over several days. If the host is already infected with the Lyme disease spirochete from previous tick bites, the larva will likely become infected as well. In this way, infected hosts in the wild (primarily white-footed mice, which exist in large numbers in Lymeendemic areas of the northeast and upper mid-west) serve as spirochete reservoirs, infecting ticks that feed upon them. Other mammals and ground-feeding birds may also serve as reservoirs. Because deer tick larvae are not born infected, it is believed that they cannot transmit Lyme disease to their human hosts. Instead, "reservoir" hosts, as mentioned above, can infect the larvae. Having already fed, an infected larva will not seek another host, human or otherwise, until after it reaches the next stage in its life cycle. It is not completely known whether larvae, in themselves, pose a threat to humans or their pets. Nymph: Most larvae, after feeding, drop off their hosts and molt, or transform, into nymphs in the fall. The nymphs can remain active throughout the winter and early spring. In May, nymphal activity begins. Host-seeking nymphs wait on vegetation near the ground for a small mammal or bird to approach. The nymph will then latch on to its host and feed for 4 or 5 days, engorging with blood and swelling to many times its original size. If previously infected during its larval stage, the nymph may transmit the Lyme disease spirochete to its host. If not previously infected, the nymph may become infected if its host carries the Lyme disease spirochete from previous infectious tick bites. In highly endemic areas of the northeast, at least 25% of nymphs have been found to harbor the Lyme disease spirochete. Also humans are the hosts often that come into contact with infected nymphs during their peak spring and summer activity. Although the nymphs' preferred hosts are small mammals and birds, humans and their pets are suitable substitutes. Because nymphs are about the size of a poppy seed, they often go unnoticed until fully engorged, and are therefore responsible for the majority of human Lyme disease cases. Adult: Once engorged, the nymph drops off its host into the leaf litter and molts into an adult. These adults actively seek new hosts throughout the fall, waiting up to 3 feet above the ground on stalks of grass or leaf tips to latch onto deer (its preferred host) or other larger mammals (including humans, dogs, cats, horses, and other domestic animals). Peak activity for adult deer ticks occurs in late October and early November. Of adults sampled in highly endemic areas of the northeast, at least 50% have been found to carry the Lyme disease spirochete. As winter closes in, adult ticks unsuccessful in finding hosts take cover under leaf litter or other surface vegetation, becoming inactive when covered by ice and snow. Generally, winters in the northeast and upper mid-west are cold enough to keep adult ticks at bay until late February or early March but not when temperatures begin to rise. At this time, they resume the quest for hosts in a lastditch effort to obtain a blood meal allowing them to mate and reproduce. This second activity peak typically occurs in March and early April. Adult female ticks that attach to deer, whether in the fall or spring, feed for approximately one week. Males feed only intermittently. Mating may take place on or off the host, and is required for the female's successful completion of the blood meal. The females then drop off the host, become gravid, lay their eggs underneath leaf litter in early spring, and die. Each female lays approximately 3,000 eggs. The eggs hatch later in the summer, beginning the two-year cycle anew. TOPIC: INSECTA, DIPTERA Insects Insects are invertebrate animals of the Class Insecta, the largest and (on land) most widely distributed taxon within the Phylum Arthropoda. Insects comprise the most diverse group of animals on the earth, with over 800,000 species described— more than all other animal groups combined: "Indeed, in no one of her works has Nature more fully displayed her exhaustless ingenuity," Pliny exclaimed. Insects may be found in nearly all environments on the planet, although only a small number of species have adapted to life in the oceans where crustaceans tend to predominate. There are approximately 5,000 dragonfly species, 2,000 praying mantis, 20,000 grasshopper, 170,000 butterfly and moth, 120,000 fly, 82,000 true bug, 350,000 beetle, and 110,000 bee and ant species. Estimates of the total number of current species, including those not yet known to science, range from two to thirty million, with most authorities favouring a figure midway between these extremes. The study of insects is called entomology. Relationship to other arthropods: A few smaller groups with similar body plans, such as springtails (Collembola), are united with the insects in the Subphylum Hexapoda. The true insects (that is, species classified in the Class Insecta) are distinguished from all other arthropods in part by having ectognathous, or exposed, mouthparts and eleven abdominal segments. Most species, but by no means all, have wings as adults. Terrestrial arthropods, such as centipedes, millipedes, scorpions and spiders, are sometimes confused with insects due to the fact that both have similar body plans, sharing (as do all arthropods) a jointed exoskeleton. Morphology and development: Insects range in size from less than a millimeter to over 18 centimeters (some walking sticks) in length. Insects possess segmented bodies supported by an exoskeleton, a hard outer covering made mostly of chitin. The body is divided into a head, a thorax, and an abdomen. The head supports a pair of sensory antennae, a pair of compound eyes, and a mouth. The thorax has six legs (one pair per segment) and wings (if present in the species). The abdomen has excretory and reproductive structures. Insects have a complete digestive system. That is, their digestive system consists basically of a tube that runs from mouth to anus, contrasting with the incomplete digestive systems found in many simpler invertebrates. The excretory system consists of Malpighian tubules for the removal of nitrogenous wastes and the hindgut for osmoregulation. At the end of the hindgut, insects are able to reabsorb water along with potassium and sodium ions. Therefore, insects don't usually excrete water with their feces, a fact, which allows them to store water in the body. This process of reabsorption enables them to withstand hot, dry environments. Most insects have two pairs of wings located on the second and third thoracic segments. Insects are the only invertebrate group to have developed flight, and this has played an important part in their success. The winged insects, and their secondarily wingless relatives, make up the subclass Pterygota. Insect flight is not very well understood, relying heavily on turbulent atmospheric effects. In more primitive insects it tends to rely heavily on direct flight muscles, which act upon the wing structure. More advanced flyers, which make up the Neoptera, generally have wings that can be folded over their back, keeping them out of the way when not in use. In these insects, the wings are powered mainly by indirect flight muscles that move the wings by stressing the thorax wall. These muscles are able to contract when stretched without nervous impulses, allowing the wings to beat much faster than would be otherwise possible. Insects use tracheal respiration in order to transport oxygen through their bodies. Openings on the surface of the body called spiracles lead to the tubular tracheal system. Air reaches internal tissues via this system of branching trachea. The circulatory system of insects, like that of other arthropods, is open: the heart pumps the hemolymph through arteries to open spaces surrounding the internal organs; when the heart relaxes, the hemolymph seeps back into the heart. Insects hatch from eggs, and undergo a series of moults as they develop and grow in size. This manner of growth is necessitated by the exoskeleton. Moulting is a process by which the individual escapes the confines of the exoskeleton in order to increase in size, then grows a new outer covering. In most types of insects, the young, called nymphs, are basically similar in form to the adults (an example is the grasshopper), though wings are not developed until the adult stage. This is called incomplete metamorphosis. Complete metamorphosis distinguishes the Endopterygota, which includes many of the most successful insect groups. In these species, an egg hatches to produce a larva, which is generally worm-like in form. The larva grows and eventually becomes a pupa, a stage sealed within a cocoon or chrysalis in some species. In the pupal stage, the insect undergoes considerable change in form to emerge as an adult, or imago. Butterflies are an example of insects that undergoe complete metamorphosis. Behaviour: Many insects possess very refined organs of perception. In some cases, their senses can be more capable than humans. For example, bees can see in the ultraviolet spectrum, and male moths have a specialized sense of smell that enables them to detect the pheromones of female moths over distances of many kilometers. Social insects, such as the ant and the bee, are the most familiar species of eusocial animal. They live together in large well-organized colonies that are so tightly integrated and genetically similar the colonies are sometimes considered superorganisms. Roles in the environment and human society: Many insects are considered pests by humans, because they transmit diseases (mosquitoes, flies), damage structures (termites), or destroy agricultural goods (locusts, weevils). Many entomologists are involved in various forms of pest control, often using insecticides, but more and more relying on methods of biocontrol. Although pest insects attract the most attention, many insects are beneficial to the environment and to humans. Some pollinate flowering plants (for example wasps, bees, butterflies, ants). Pollination is a trade between plants, which need to reproduce, and pollinators which receive rewards of nectar and pollen. A serious environmental problem today is the decline of populations of pollinator insects, and a number of species of insects are now cultured primarily for pollination management in order to have sufficient pollinators in the field, orchard or greenhouse at bloom time. Insects also produce useful substances such as honey, wax, lacquer or silk. Honeybees, have been cultured by humans for thousands of years for honey, although contracting for crop pollination is becoming more significant for beekeepers. The silkworm has greatly affected human history as silk-driven trade established relationships between China and the rest of the world. Fly larvae (maggots) were formerly used to treat wounds to prevent or stop gangrene, as they would only consume dead flesh. This treatment is finding modern usage in some hospitals. Insect larvae of various kinds are also commonly used as fishing bait. In some parts of the world, insects are used for human food ("Entomophagy"), while being a taboo in other places. There are proponents of developing this use to provide a major source of protein in human nutrition. Since it is impossible to entirely eliminate pest insects from the human food chain, insects already are present in many foods, especially grains. Most people do not realize that food laws in many countries do not prohibit insect parts in food, but rather limit the quantity. According to cultural materialist anthropologist Marvin Harris, the eating of insects is taboo in cultures that have protein sources that require less work like farm birds or cattle. Many insects, especially beetles, are scavengers, feeding on dead animals and fallen trees, recycling the biological materials into forms found useful by other organisms. The ancient Egyptian religion adored beetles and represented them as scarabeums. Although mostly unnoticed by most humans, arguably the most useful of all insects are insectivores, those that feed on other insects. Many insects, such as grasshoppers can potentially reproduce so fast that they could literally bury the earth in a single season. However there are hundreds of other insect species that feed on grasshopper eggs, and some that feed on grasshopper adults. This role in ecology is usually assumed to be primarily one of birds, but insects, though less glamorous, are much more significant. For any pest insect one can name, there is a species of wasp that is either a parasitoid or predator upon that pest, and plays a significant role in controlling it. Human attempts to control pests by insecticides can backfire, because important but unrecognized insects already helping to control pest populations are also killed by the poison, leading eventually to population explosions of the pest species. Cockroaches Cockroaches are insects of the order Blattodea (the name Blattaria is also seen). The names of the order are derived from Greek blatta, meaning "cockroach". There are roughly 3,500 species in 6 families. Cockroaches exist worldwide, with the exception of the polar regions and in elevations above 2,000 m (6,500 ft). Among the most well-known species are the American cockroach, Periplaneta americana, which is about 3 cm long, and the German cockroach, Blattella germanica, about 1.5 cm long. Tropical cockroaches are often much bigger. When infesting buildings, cockroaches are considered pests. The earliest fossils of cockroaches are from the Carboniferous period between 354–295 million years ago. Biology: Cockroaches are generally either scavengers or omnivores. The exception to this is the wood eating Cryptocercus species found in China and the United States. Although they are incapable of digesting the cellulose themselves, they have a symbiotic relationship with a protozoan that digests the cellulose, allowing them to extract the nutrients. In this, they are similar to termites. They are most common in tropical and subtropical climates. Some species are in close association with human dwellings and widely found around garbage or in the kitchen. Female cockroaches are sometimes seen carrying egg cases on the end of their abdomen; the egg case of the German Cockroach holds about 30–40 long, thin eggs, packed like frankfurters in the case called an ootheca. The eggs hatch from the combined pressure of the hatchlings gulping air and are initially bright white nymphs that continue inflating themselves with air and harden and darken within about four hours. Their transient white stage while hatching and later while molting has led to many individuals claiming to have seen albino cockroaches. A female German cockroach carries an egg capsule containing around 40 eggs. She drops the capsule prior to hatching. Development from eggs to adults takes 3–4 months. Cockroaches live up to a year. The female may produce up to eight egg cases in a lifetime. In other words, in favorable conditions it can produce 300–400 offspring. A regular cockroach, however, can produce an extremely high number of eggs in her lifetime. She lays up to 100 eggs in each egg sac. She only needs to be impregnated once to be able to lay eggs for the rest of her life, allowing one single cockroach to lay over a million eggs in her lifetime. The world's largest cockroach is the Australian giant burrowing cockroach, which can grow to 9 cm in length and weigh more than 30 grams. Comparable in size is the giant cockroach Blaberus giganteus, which grows to a similar length but is not as massive. Cockroaches are mainly nocturnal, and will run away when exposed to light. A peculiar exception is the Oriental Cockroach which is attracted to light, thus making it a far more annoying pest. Roaches are actually very clean insects, even though they eat garbage. They are called the custodians of nature. They only live in houses where there are crumbs to eat or the garbage can is uncovered. They lay eggs inside the house's hollow walls. The cockroach is also one of the hardiest insects on the planet, capable of living for a month without food and remaining alive headless for up to a week. It can also hold its breath for 45 minutes and has the ability to slow down its heart rate. Cockroaches also have a very high resistance to radiation. Select species Periplaneta americana, American cockroach Eurycotis floridana, Florida woods cockroach Blatta orientalis, Oriental cockroach Blattella germanica, German cockroach Blattella asahinai, Asian cockroach Pycnoscelus surinamensis, Surinam cockroach Supella longipalpa, Brown-banded cockroach Periplaneta australasiae, Australian cockroach Periplaneta fuliginosa, Smokybrown cockroach Parcoblatta pennsylvanica, Pennsylvania woods cockroach Periplaneta brunnea, Brown cockroach Behavior: New research being conducted at the University of Florida shows that cockroaches leave chemical trails in their feces. Other cockroaches will follow these trails to discover sources of food, water, and where other cockroaches are hiding. One of the major implications of this research is a new technique in cockroach pest control. Cockroaches could be potentially removed from a home by leaving a chemical trail that leads away from the home. Miscellaneous: The largest known cockroach by wingspan is a Megaloblatta longipennis, with an 18-cm wingspan. The largest by weight is a 50g Macropanesthia rhinoceros. The smallest species is Attaphila fungicola, reaching only 4 mm. Heteroptera Heteroptera (also called true bugs) is a suborder of the order Hemiptera. There are 25,000 known species in over 60 families. The name Heteroptera comes from their forewings having both membranous and hard portions. Suborder Heteroptera includes 25,000 known species in over 60 families. Hemiptera is an order of insects, comprising some 67,500 known species in two suborders, Heteroptera and Homoptera. Originally the Homoptera were treated as a separate order. Members of the Hemiptera, and of the Heteroptera in particular, are sometimes called "true bugs". The name "heteroptera" comes from their forewings having both membranous and hard portions. It is also this, which gives the order its name, hemiptera, coming from the Greek for half-wing. Species of order Hemiptera occur worldwide; they are distinguished from all other insects by both adults and nymphs having piercing and sucking mouthparts housed in a long "beak". These are used mostly to feed on plant juices, but some species are adapted to suck blood from animals or other insects. Bedbugs Bedbugs (or bed bugs) are small nocturnal insects of the family Cimicidae that live by hematophagy, feeding on the blood of humans and other warm-blooded hosts. The common bedbug (Cimex lectularius) is the best adapted to human environments. It is found in temperate climates throughout the world and has been known since ancient times. Bedbugs are small, brown, wingless parasites that feed on blood (Fig. 39). Even though they cannot fly, they are not easily seen, as they are less than a quarterof-an-inch (half a centimeter) long and often hide during the day. While bedbugs prefer to bite humans, they will also feed from other mammals if necessary. Bedbugs get their name from the fact they often live in unsanitary mattresses and bedding, but they can be found in other places such as carpets and cracks in walls. Although they are often found in dirty accommodation where poor sanitation is a problem, bedbugs have been known to travel in a person’s clothing or luggage to other locations. One sign of bedbugs is finding spots of blood in or around beds. Bedbugs typically leave tiny, itchy bites in orderly rows on a body. Some may notice that rooms that have many bedbugs have a sweet smell. Bedbugs are mainly prevented by good sanitation and frequent cleaning, such as regular house cleaning and washing of bedding. As bedbugs are found worldwide, travelers abroad should also be watchful for signs of infestation. Travelers in rustic or less-developed areas can reduce their chances of being bitten by staying in reputable, well-maintained accommodations that appear clean. Travelers can also reduce chances of an infestation at home by washing all clothing and luggage upon returning home. Bedbugs can leave itchy bites that typically heal over a few days. The itch common to the bites can usually be solved with a variety of common bug-bite remedies. A doctor should be consulted in more serious cases. Bedbug infestations can be difficult to eliminate, as the pests can hide in a number of places. Mattresses and carpets suspected to contain bedbugs can be thoroughly vacuumed to remove the pests, with mattresses then being covered and left in a sunny place for as long as possible. Bedding and clothing can be washed thoroughly in hot water. However, professional pest control should also be considered, especially in larger or repetitive cases. Anoplura Sucking lice (Anoplura) have around 500 species and represent the smaller of the two traditional suborders of lice. The Anoplura are all blood-feeding ectoparasites of mammals. They can cause localized skin irritations and are vectors of several blood-borne diseases. At least three species of Anoplura are parasites of humans. Pediculus humanus is divided into two subspecies, Pediculus humanus humanus, or the body louse, sometimes nicknamed "the seam squirrel" for its habit of laying of eggs in the seams of clothing, and Pediculus humanus capitis, or the head louse. Phthirus pubis (the pubic louse) is the cause of the embarrassing condition known as crabs. Head lice Head lice (Pediculus humanus capitis) are one of the many varieties of sucking lice (singular "louse") specialized to live on different areas of various animals. As the name implies, head lice are specialized to live among the hair present on the human head and are exquisitely adapted to living mainly on the scalp and neck hairs of their human host. Lice present on other body parts covered by hair are not head lice but are either Pubic lice (Pthirus pubis) or Body lice (Pediculus humanus humanus). Description: The adult head louse resembles a miniature ant that appears flat when viewed from the side through a strong magnifying glass (Fig. 40). Head lice have a head, thorax and abdomen with six legs, but their two front legs are very large in order to grab onto the hair shafts. Head lice are tan to greyish-white in color. Life cycle: Lice eggs on the hair very close to the scalp are the primary sign of an active infestation. The female louse glues her eggs, sometimes called "nits", which look like tiny white beads, to hair shafts very close to the scalp. Eggs are very small, about the size of a period (full stop) in normal printing. Eggs may appear yellowish, brownish or greyish, but almost always lighter colored. Eggs normally undergo 7-9 day incubation before hatching as a baby nymph. Classically, a louse egg does not become a "nit" until after it has completed its incubation stage, thus leaving a "nit". A "nit" is either the empty shell remaining after the nymph has departed or the dead egg that remains if incubation was not successful. Dead eggs will appear dark, or raisin-like, as they dry out. "Nits" are usually found one-half inch or more away from the scalp and are not considered a sign of an active infestation. There are three nymph instar stages as the baby louse matures, with the louse shedding its exoskeleton at the end of each stage, as it gets larger. The nymph stage typically lasts 10 to 12 days. Whether a louse is male or female is not apparent until they are nearly mature. Fertilization of eggs takes place once as the female reaches the mature stage. The female can then lay 3-7 eggs each day for the next 28 to 30 days, her normal life span. There are three main stages in the life of a head louse: the nit, the nymph, and the adult. Nit: Nits are head lice eggs. They are hard to see and are found firmly attached to the hair shaft. They are oval and usually yellow to white. Nits take about 1 week to hatch. Nymph: The nit hatches into a baby louse called a nymph. It looks like an adult head louse, but is smaller. Nymphs mature into adults about 7 days after hatching. To live, the nymph must feed on blood. It metamorphoses 3 times before it reaches the adult stage. Adult: Females lay nits (a few hundreds of eggs); they are usually larger than males. To live, adult lice need to feed on blood. If the louse falls off a person, it dies within 1-2 days. Symptoms: The louse feeds on human blood, and the bite causes itching. Bites can become secondarily infected; scratching may break the skin and help cause this secondary infection. The most common symptom is itching of the scalp. Head lice are normally spread by close contact but can also be spread by sharing clothes. Treatment: The most common Western treatment is with chemical insecticides such as pyrethrin, however, there is increasing controversy over possible toxic side effects. For more information as well as alternate treatments, see Treatment of human head lice. Crab Lice Class: Insecta Order: Phithiraptera Genus: Phthirus Introduction: The crab louse is 1-2mm long and distinguished by a square, undifferentiated body and massive claws on the two posterior sets of forelegs. These claws are able to grasp both pubic and facial hair (including eyelashes), and allow the louse to remain tightly bound to the host. They are spread mostly by sexual contact, but may also be transmitted through fomites. Life Cycle: The life cycle of Phthirus is very similar to Pediculus. Females lay bundles of eggs on the coarse pubic hairs and dense facial hairs of humans. The crab lice proceed through a cycle similar to the head and body lice, with the nymphal stage proceeding several days longer. Phthirus are less active than Pediculus, but similarly cannot survive for very long without a host and blood meals. Disease: There appears to be very little evidence of disease transmission by Pthirus, but have the ability to cause severe localized allergic reactions during infestations. Fleas Fleas are external parasites, living by hematophagy off the blood of mammals and birds. Note: There is also a genus of Protozoa named Siphonaptera. Some well known flea species include: Cat Flea (Ctenocephalides felis), Dog Flea (Ctenocephalides canis), Nothern Rat flea (Nosopsyllus fasciatus), Oriental Rat Flea (Xenopsylla cheopis). In most cases fleas are just a nuisance to their hosts, but some people and some animals suffer allergic reactions to flea saliva resulting in rashes. Flea bites generally result in the formation of a slightly-raised swollen itching spot with a single puncture point at the center. However, fleas can transmit disease. One devastating example of this was the bubonic plague, transmitted between rodents and humans. Murine typhus (endemic typhus) fever, and in some cases tapeworms can also be transmitted by fleas. Life cycle Fleas pass through a complete life cycle consisting of egg, larva, pupa and adult (Fig. 41). Completion of the life cycle from egg to adult varies from two weeks to eight months depending on the temperature, humidity, food, and species. Normally after a blood meal, the female flea lays about 15 to eggs per day – up to 600 in its lifetime – usually on the host (dogs, cats, rats, rabbits, mice, squirrels, chipmunks, raccoons, opossums, foxes, chickens, humans, etc.). Eggs loosely laid in the hair coat drop out almost anywhere, especially where the host rests, sleeps or nests (rugs, carpets, upholstered furniture, cat or dog boxes, kennels, sand boxes, etc.). Eggs hatch between two days to two weeks into larvae found indoors in and along floor cracks, crevices, along baseboards, under rug edges and in furniture or beds. Outdoor development occurs in sandy gravel soils (moist sand boxes, dirt crawlspace under the house, under shrubs, etc.) where the host may rest or sleep. Sand and gravel are very suitable for larval development, which is the reason fleas are erroneously called "sand fleas." Larvae are blind, avoid light, pass through three larval instars and take a week to several months to develop. Their food consists of digested blood from adult flea feces, dead skin, hair, feathers, and other organic debris; larvae do not suck blood. Pupae mature to adulthood within a silken cocoon woven by the larva to which pet hair, carpet fiber, dust, grass cuttings, and other debris adheres. In about five to fourteen days, adult fleas emerge or may remain resting in the cocoon until the detection of vibration (pet and people movement), pressure (host animal lying down on them), heat, noise, or carbon dioxide (meaning a potential blood source is near). Most fleas overwinter in the larval or pupal stage with survival and growth best during warm, moist winters and spring. Flea bites can be treated with Calamine Lotion or 0.5-1% hydrocortisone cream. Lufenuron is a veterinary medicine that attacks the larval fleas ability to produce chitin. Disease: Fleas are a general nuisance, often biting humans on exposed surfaces resulting in discomfort. Flea-bites induce pruritic papular urticaria commonly on the unprotected lower leg of women and all over the body of children who have intimate animal contact; a generalised allergic response may occur. Certain fleas, notably the rat fleas, spread plague (Yersinia pestis) and murine typhus (Rickettsia typhi), and serve as intermediate hosts for species of tapeworm (Hymenolepis sp.). Cat and dog fleas serve as intermediate hosts for another common tapeworm (Dipylidium caninum), which can be spread to humans, especially children with exposure to pet animals. Pulex irritans is not a major vector of disease but may play a minor role in the transmission of plague. Infection is often spread by the bite alone, but can also potential be transmitted through fecal abrasion. Tunga penetrans does not transmit disease to humans, but females will burrow into host skin. The pinpoint lesion enlarges to pea-size within 2 weeks necessitating removal of the gravid female using a pin, a needle or a sliver of bamboo. This may potentially lead to a secondary bacterial infection. Control and Treatment: Control of fleas is generally mediated through insecticidal powders and aerosols. If outbreaks of murine typhus or plague occur steps to control the rodent populations in the affected area may be employed. Diptera Diptera are insects in which the hind wings are reduced to halteres. The study of the diptera is called dipterology. Synonyms and common names: Flies, mosquitoes, gnats and midges are the species in this order. In compound names containing "fly" for members of this order, the name is written as two words as in "crane fly". For insects that are members of other orders the name is written as a single word as in “butterfly”. Subdivision: About 85,000 species of insect have only two wings and are classified as members of Diptera. There are two generally accepted sub-orders of Diptera. The Nematocera are usually recognized by their elongated bodies and feathery antennae as represented by mosquitoes and crane flies. The Brachycera tend to have a more roundly proportioned body and very short antennae. Beyond that, considerable revision in the taxonomy of the flies has taken place since the introduction of modern cladistic techniques, and much remains uncertain. The secondary ranks between the sub-orders and the families are more out of practical considerations than out of any strict respect for phylogenetic classifications. (Modern cladists tend to spurn the use of any rank names.) Several of the classifications used now in this article remain paraphyletic groupings; this is particularly notable in the Orthorrapha. Mosquitoes Class: Insecta Order: Diptera Genus: Anopheles, Aedes, Culex Introduction: Mosquitoes are small with a clearly demarcated body and very long slender legs. The head contains a large pair of kidney shaped compound eyes, a pair of antennae, and a single long proboscis for feeding. The thorax, abdomen and wings are often covered with scales. Differential colouration and pattern of these scales provides a means of visually distinguishing species. The large wings are folded over the segmented abdomen, which generally appears brown-black and slender but turns a bright red and swells following feeding. Mosquitoes may be classified as Anopheline (Anopheles) or Culicine (Aedes, Culex). The antennae of male mosquitoes are plumose (many feathery hairs); females are pilose (few spidery hairs). The male Anopheline palps are long and clubbed; those of the male Culicine are long but not clubbed. The female Anopheline palps are long; the female Culicine are short. If a mosquito is incorrectly sexed, a female Anopheline may be confused with a male Culicine (Fig. 42). Anopheles Mosquitoes Class: Insecta Order: Diptera Genus: Anopheles General Characteristics: Anopheles mosquitoes are characterised by dark and pale scale blocks arranged on their wings. They have palps that are of equal length to the proboscis, which appear terminally clubbed in males. Anopheles always rest at an angle when standing on surfaces and preferring to feed at twilight or night. Breeding sites are varied but Anopheles prefer unpolluted fresh or saltwater. Life Cycle: Anopheles lay 50 to 200 dark colour eggs in aquatic environments, and hatch in several days to several weeks depending on the external temperature. Anopheles larvae have a dark brown head and 6-7 anterior segments covered with dorsal palmate hairs. Accessory tergal plates are present on the dorsal side of segments 1-10 and two sets of anal papillae emerge from the last abdominal segment. There are four larval instars that survive by filter feeding and breathing oxygen through their spiracles. Anopheles larvae occur throughout many different habitats including both permanent marshes and swamps, and temporary locations such as pots filled with water. In general Anopheles prefer to inhabit clean habitats. The larval period lasts about a week, but may be extended depending on the environmental conditions. The pupa is comma shaped with a set of trumpet shaped breathing tubes. The abdomen is covered with setae, and segments 2-7 have distinct spines. The pupal period may last a few days to weeks depending on the temperature. Diseases Malaria: Anopheles are vectors of malaria, Bancroftian and Brugian filariasis and of multiple arboviruses (dengue fever; yellow fever; encephalitides and haemorrhagic fevers). Malaria is caused by Plasmodium falciparum, P. vivax, P. malariae and P. ovale. Transmission of the disease occurs in virtually all of tropical Africa, Central and South America, and the Middle and Far East. South East Asia is a particular problem due to multiple drug resistance. P. falciparum is found in Africa and other tropical countries as well as in subtropics. P. malariae has a low prevalence in both tropics and subtropics. P .vivax is the most widespread in temperate regions and subtropics but may also be found in the tropics. P. ovale has a low prevalence in West Africa. In Africa alone, 370 million people live in endemic areas. P.vivax causes benign tertian malaria (43% of cases) and P. falciparum results in malignant tertian or sub-tertian malaria and pernicious malaria (50% of cases). P .ovale (mild tertian malaria, 1% of cases) and P. malariae (quartan malaria, 7% of cases) contribute a small percentage of malarial cases. Clinical features including fever and chills are due to the host inflammatory response and are associated with rupture of erythrocytic schizonts. Fever presents in three stages – a) Cold: rigors and fever lasting 15 minutes to 1 hour; b) Hot: the patient is flushed with tachycardia and is pyrexial (40C) for 2-6 hours; c) Sweating: the temperature falls (over 2-4 hours). Each paroxysm lasts 8-12 hours in total. All erythrocytes containing a trophozoite will be destroyed within 48-72 hours. Periodic fever often takes more than 7 days to develop, and anaemia can be haemolytic or due to toxic marrow suppression. Splenomegaly occurs in all malaria: it may be acute or chronic (+/- hypersplenism). Jaundice may be haemolytic and/or hepatic (only P. falciparum). In addition, there may be headache, myalgia, arthralgia, diarrhoea and vomiting. Plasmodium falciparum is the most virulent form (invades mature and immature RBCs) and is often fatal if untreated. Blood schizogony takes place in deep capillaries and micro-circulatory failure can occur in individuals with little immunity to malaria. It does not relapse but recrudescence may occur. The time between paroxysms is 48 hours but fever may last for 24-36 hours. Very rapid progression and complications include diarrhoea and vomiting; delirium; coma; convulsions; renal failure, including haemoglobinuria (blackwater fever); jaundice; pulmonary oedema; hypoglycaemia and abortion. Cerebral malaria often results in delirium, disorientation, stupor, coma, convulsions and death. P. vivax / ovale exhibit 48 hours between paroxysms; relapses may occur up to 8 years after primary infection and only infects immature RBCs of those with Duffy blood group. Plasmodium malariae generally results in72 hours between paroxysms, only infects older RBCs, and recrudescence may occur decades after primary infection. The global malaria situation is serious and becoming worse: 300-500 million clinical cases occur annually. 1.5 – 2.7 million people die of malaria each year with approximately one million deaths among children under five years of age are attributed to malaria alone or in combination with other diseases. Countries in tropical Africa account for more than 90% of the total malaria incidence and the great majority of malaria deaths (WHO data). The death toll of African children with malaria is expected to double by 2010, conceivably reaching 4 million deaths per year. Many factors influence the epidemiology of this disease including: breeding habits of the various mosquito vectors; agricultural practices; economic conditions; industrialisation and pesticide use. Increasing air-traffic from malaria endemic areas has led to the possibility of malaria developing in non-endemic areas where the mosquito vector has been imported onboard aircraft. Filariasis Anopheline mosquitoes also transmit the filarial worms Wuchereria bancrofti, Brugia malayi and Brugia timori. Wuchereria bancrofti is the main cause of "elephantiasis" (Bancroftian filariasis) and the most widely distributed filarial parasite of man. The adults live in the lymphatic system, and can survive for 30 years or more. Once they have mated they produce a pre-larval form, the microfilaria. Both the adults and the microfilaria may play a role in generating the symptoms and signs. Microfilaria measure 240-300m in length and 7-10m in width. They are sheathed (derived from ovum membrane) and nuclei terminate 15-20m proximal to the pointed tail. There are fewer, more distinct nuclei than in other species and there are less body curves. Adult worms are slender and white (males 4 cm; females stout and 10 cm in length). Initial infection with Wuchereria is usually asymptomatic. There may be recurrence of attacks of "cellulitis" affecting the limbs, breast, scrotum or elsewhere. Infection is associated with fever, lymphangitis, lymphadenopathy and occasionally abscess formation. These initially settle but later on the tissues eventually become oedematous and hypertrophied. Further effects may include scrotal involvement and hydrocoele, which can lead to scrotal enlargement and lymph scrotum. This is "elephantiasis" and is associated with dermal hypertrophy, verrucous changes and the rupture of lymph varices into various sites. Brugian (Malayan) filariasis is less widespread, less common and less serious than its Bancroftian counterpart. The life cycle is identical to that of Wuchereria bancrofti with Brugia malayi limited to Asia and B.timori restricted to Indonesia. Infection results in lymphadenopathy involving most frequently the inguinal area, lymphoedema normally below the knee, eosinophilia, and in rare cases chyluria. Treatment and Control Malaria: If the infective species is not known, or the infection is known to be mixed, initial treatment should be with quinine, mefloquine or rarely halofantrine. Falciparum (malignant) malaria is often resistant to chloroquine and should be treated with quinine, mefloquine, halofantrine, quinidine or pyrimethamine-sulphadoxine. Benign malaria (P. vivax) should be treated with chloroquine although resistance has been reported from New Guinea. Malarial prophylaxis is relative and not absolute. The UK Consensus Group on Malaria Prophylaxis (1997) recommend mefloquine for UK travellers to West, Central and East Africa for periods of greater than 2 weeks and for travellers to specific areas within south-east Asia: prophylaxis should be commenced 2 weeks before departure. Doxycycline can be used in older children and adults who cannot tolerate mefloquine. Prevention is most dependent upon coverage of exposed skin and the use of insect repellent, mosquito nets impregnated with permethrin and correct prophylaxis. The vector may be controlled by water clearance programs, house spraying (DDT) and destruction of breeding areas. Drug resistance to DDT and ethical resistance to its use have limited its effectiveness. Natural immunity involves both antibody and cellmediated systems and appears to require frequent boosting; antigens from different stages of the parasite's life cycle will be important in vaccine development. Filariasis Diethylcarbamazine (DEC) kills microfilaria. Ivermectin suppresses microfilaria production but its overall effectiveness remains untried and elephantiasis can be treated surgically. Control measures comprise draining of mosquito breeding sites and killing larvae. Many mosquitoes are resistant to insecticides but mosquito repellents and nets are effective. The infective pool may be reduced by periodic mass treatment with DEC. Brugia malayi is more susceptible to diethylcarbamazine (DEC) than is Wuchereria bancrofti. Anopheline larvae may be suffocated in their breeding sites but culicine larvae (Mansonia sp.) derive oxygen from plants and are not amenable to such measures. Control depends upon the use of mosquito nets and periodic mass treatment. Aedes mosquitoes Class: Insecta Order: Diptera Genus: Aedes General Characteristics: Aedes can generally be distinguished by patterns of black and silvery scales present on the abdomen and thorax. The legs appear to have black and white rings along their length. The wings are generally covered with black scales. Aedes breed in marshes and other wetland areas and have a worldwide distribution. Life Cycle: Female Aedes lay eggs on damp areas such mud, detritus, clay and rock. The eggs are very robust and can survive desiccation and other environmental pressures. The eggs hatch in waves depending on the environmental cues. Aedes larvae have a stout barrel shaped siphon with one pair of subventral tufts. There are three pairs of setae on the ventral brush, and large setae are not present on the abdominal segments. Disease: Aedes are vectors of Bancroftian filariasis and arboviruses such as yellow fever and dengue. Wuchereria bancrofti is the main cause of "elephantiasis" (Bancroftian filariasis) and the most widely distributed filarial parasite of Man. The adults live in the lymphatic system, and can survive for 30 years or more. They copulate and generate a pre-larval form, the microfilaria. Both the adults and the microfilaria may play a role in generating the symptoms and signs. Microfilaria measure 240-300 m in length by 7-10 m in width. They are sheathed (derived from ovum membrane) and nuclei terminate 15-20 microns proximal to the pointed tail. There are fewer, more distinct nuclei than in other species and there are less body curves. Adult worms are slender and white (males 4 mm; females stout and 10 mm in length). Initial infection with Wuchereria is usually asymptomatic. There may be recurrence of attacks of "cellulitis" affecting the limbs, breast, scrotum or elsewhere. Infection is associated with fever, lymphangitis, lymphadenopathy and occasionally abscess formation. These initially settle but later on the tissues eventually become oedematous and hypertrophied. Further effects may include scrotal involvement and hydrocoele, which can lead to scrotal enlargement and lymph scrotum. This is "elephantiasis" and is associated with dermal hypertrophy, verrucous changes and the rupture of lymph varices into various sites. Yellow fever and dengue haemorrhagic fever are serious viral infections spread by the Aedes mosquito. Dengue is now the most important mosquito borne virus, with global infection increasing. Control and Treatment: In general the most effective control for Culicine mosquitoes are also repellents and fine screening or netting. Treatment with insecticides will also serve to reduce the vector population, but increased problems are encountered with Culicines because they also feed during the daytime. If filarial infection occurs treatment with Diethylcarbamazine (DEC) will kill microfilaria. Ivermectin suppresses microfilaria production but its overall effectiveness remains untried and elephantiasis can be treated surgically. Culex mosquitoes Class: Insecta Order: Diptera Genus: Culex General Characteristics: Culex are distinguished by their lack of colouration and feature. The thorax, abdomen, legs and wings are often covered with brownblack scales giving a generally dark appearance. The abdomen may occasionally also have white scales arranged in segments. Culex breeds mainly in aquatic habitats, often in areas containing large quantities of organic waste. Life Cycle: Female Culex lay dark brown eggs in characteristic clumps of approximately 300 eggs. As mentioned above these eggs are often found in organic waste deposits or polluted waters. Culex larvae have a long and narrow siphon with more than one pair of subventral tufts. Disease: Culex mosquitoes are vectors of Bancroftian filariasis throughout Africa, but most importantly arboviruses such as Japanese encephalitis. Encephalitis occurs throughout the world, with Culex acting as an important vector for spread and infection. Culex mosquitoes are similar to the Culicine and Aede mosquitoes, but prefer to bite at night and breed in organic refuse. Control and Treatment: Culex mosquitoes are most easily controlled by improving sanitation and removing static water sources from the affected area. In general the most effective control for Culex mosquitoes are also repellents and fine screening or netting. Treatment with insecticides will also serve to reduce the vector population, but increased problems are encountered with Culicine mosquitoes because they also feed during the daytime. If filarial infection occurs treatment with Diethylcarbamazine (DEC) will kill microfilaria. Ivermectin suppresses microfilaria production but its overall effectiveness remains untried and elephantiasis can be treated surgically. Phlebotomus Phlebotomus is a genus of flies, or diptera, that generally includes "sand flies" (Fig. 43). In the Old World, Phlebotomus sand flies are primarily responsible for the transmission of leishmaniasis, an important parasitic disease. Leishmaniasis is generally transmitted in the New World by sand flies of the genus Lutzomyia. The parasite itself is a species of the genus leishmania, a protozoan. The disease normally finds a mammalian reservoir in rodents and other small animals such as canids and hyraxes. The sand fly carries the leishmania protozoa from infected animals after feeding, thus transmitting the disease. Black Fly A Black Fly (sometimes called a Buffalo Gnat or Turkey Gnat) is any member of the family Simuliidae of the Culicomorpha infraorder. There are over 1800 known species of Black Flies (of which 11 are extinct). Like mosquitoes, to which they are related, most Black Flies gain nourishment by sucking the blood of other animals. They are usually small, black or gray, with short legs and antennae. They are a common nuisance for humans, and many US states have programs to supress the Black Fly population. They spread several diseases, including river blindness in Africa. Horse-Flies Among the world's largest flies are the horse-flies (family Tabanidae). Although not all the species in this family bite, these large, hairy flies are most often known as pests because of the painful bites many species can inflict on animals and humans (Fig. 44). They occur worldwide, being absent only at extreme northern and southern latitudes. Flies of this type are among those known sometimes as "gadflies". A type of insect, horse-flies are classified in the fly order Diptera. There are approximately 3,000 species of horse-flies known worldwide, 350 of which are found in North America. At least three subfamilies are recognised: Chrysopsinae Pangoniinae Tabaninae The genus Zophina is of uncertain placement, though it has been classified among the Pangoniinae. The two best-known types are the common horse-flies, genus Tabanus, and the deer-flies, genus Chrysops, also known as banded horse-flies because of their coloring. Both these genera give their names to subfamilies. The "Blue Tail Fly" in the eponymous song was probably a tabaninid common to the southeastern United States. Adult horse-flies feed on nectar and other plant juices, but only the females also feed on blood. Males lack the necessary mouth apparatus to do so. Most horse-flies feed on mammal blood, but some species are known to feed on birds, amphibians or reptiles. The females' primary sense for locating prey is sight, and they have large, compound eyes that serve this purpose well. The flies usually lay waiting in shady areas for prey to happen by. They are attracted to large, dark objects, and to certain animal odors and carbon dioxide. They are also attracted by motion, their eyes being well adapted to its detection. The eyes of horse-flies are generally brightly colored, and this coloration is the primary means entomologists use to sex them. A horse-fly's bite can be very painful. Unlike insects that pierce the skin, horse-flies have mouthparts that work like miniature knives, which they use to slash open the skin with a scissor-like motion. This causes the blood to seep out as the horse-fly licks it up. While some horse-flies are known to have venom, none is known to be dangerous to humans. When attacking humans, the flies generally prefer the head and upper body regions, going unnoticed until a bite is inflicted. Horse-flies are most active in hot weather, mostly in summer and autumn during the daylight hours. Most species also prefer a wet climate, which makes it easier for them to breed. Eggs are generally laid on stones close to water or on plant stems or leaves. On hatching, the larva fall into water or moist earth, feeding voraciously on invertebrates, such as snails and earthworms, and small vertebrates. Some horse-fly species are known to transmit disease and/or parasites. Chrysops is a biologic vector of Loa loa, transmitting this filariasis between humans. A common problem in some animals, though, when large flies are abundant, is blood loss. Some animals have been known to lose up to 300 ml of blood in a single day, which can severely weaken or even kill them. Citation Although the tsetse flies were responsible for transmitting sleeping sickness in most areas, occasionally an epidemic occurred in which the disease might be conveyed to cattle by direct contact with the ordinary horse fly, tanidae. This probably occurred when swarms of these flies surrounded the wretched animals. In one such epidemic some 3000 head of cattle died of trypanosomal disease in northern Rhodesia. Sir David and Lady Bruce returned to England in 1913. David Bruce reported the results achieved by this Sleeping Sickness Commission of the Royal Society in the Croonian Lectures in 1915. Musca domestica The housefly (also house-fly or house fly) (Musca domestica) is the most common fly occurring in homes and indeed one of the most widely distributed animals and the most familiar of all flies; it is a pest that can facilitate serious diseases. Physical description: The adults are 5-8 mm long. Their thorax is greyish, with four dark longitudinal lines on the back. The underside of the abdomen is yellowish. The whole body is covered with hair. They have reddish compound eyes. The females are slightly larger than the males and have a much larger space between the eyes. Like most Diptera, houseflies have only one pair of wings; the hind pair is reduced to small halteres that aid in flight stability. Characteristically, the fourth long vein of the wing shows a sharp upward bend. Species that appear similar to the housefly include: The lesser house fly (Fannia canicularis), somewhat smaller and more slender than M. domestica, fourth long vein of the wing is straight. The stable fly (Stomoxys calcitrans) looks similar to M. domestica but has a longer piercing mouthpart, used to penetrate the skin of humans and animals in order to suck blood. Life cycle: Each female fly can lay up to 500 eggs (in five batches of 100 eggs each). The eggs are white at about 1.2 m in length. Within a day, the larvae (maggots) hatch from the eggs; they live and feed in (usually dead and decaying) organic material, such as garbage or feces. They are pale whitish, 3-9 mm long, thinner at the mouth end, and have no legs. After several molts, the maggots crawl to a dry cool place and transform into pupae, colored reddish or brown and about 8mm long. The adult flies then emerge from the pupae. (This whole cycle is known as complete metamorphosis.) The adults live from half a month to a month. After having emerged from the pupae, the flies cease to grow; small flies are not young flies but the result of little food during the maggot stage. Some 36 hours after having emerged from the pupa, the female is receptive for mating. The male mounts her from the back to inject sperm. Normally the female mates only once, storing the sperm to use it repeatedly for several sets of eggs. Males are territorial: they defend a certain territory against other males and try to mount any females that enter that territory. The flies depend on warm temperatures; generally, the warmer the temperature the faster the flies will develop. In winter, most of them survive in the larval or pupa stage in some protected warm location. Some species of wasps can parasitize and kill the pupae. Typical behaviors: Houseflies can only take in liquid foods. They spit out saliva on solid foods to pre-digest it, and then suck it back in. They also throw up partially digested matter and eat it again. The flies can walk on vertical planes, and can even hang upside down from ceilings. This is accomplished with the surface tension of liquids secreted by glands near their feet. Lacking eyelids, the flies continually clean their eyes with their forelegs. Most of their taste and smell sensor cells are on hairs on their legs, this is why they also keep rubbing their legs together. Flies have a very highly-evolved evasion reaction which helps to ensure their survival. It is possible to confuse a fly´s evasion system by swatting it with two objects simultaneously from different directions. The holes in a fly swatter minimise the air current which warns the fly of being hit, whilst reducing air resistance and increasing speed of the swat. Sex determination mechanism: The housefly is an object of biological research, mainly because of one remarkable quality: the sex determination mechanism. Although a wide variety of sex determination mechanisms exists in nature (e.g. male and female heterogamy, haplodiploidy, environmental factors) the way sex is determined is usually fixed within one species. However, the housefly exhibits many different mechanisms for sex determination, such as male heterogamy (like most insects and mammals), female heterogamy (like birds) and maternal control over offspring sex. This makes the housefly one of the most suitable species to study the evolution of sex determination. Evolution: Even though the order of flies (Diptera) is much older, true houeseflies evolved in the beginning of the Cenozoic era, some 65 million years ago. They probably originated in what is today Africa and spread to Europe and Asia. They are not native to the Americas and it is quite possible that they were introduced by Christopher Columbus or other early seamen. Flies and humans: In colder climates, houseflies only occur together with humans. They have a tendency to aggregate and are difficult to dispel. They are capable of carrying over 100 pathogens, such as typhoid, cholera, Salmonella, bacillary dysentery, tuberculosis, anthrax ophthalmia, and parasitic worms. The flies in poorer and lower-hygiene areas usually carry more pathogens. Some strains have become immune to common insecticides. In art, extremely life-like houseflies have sometimes been depicted in the trompe l'oeil paintings of the 15th century. An example is the painting Portrait of a Carthusian by Petrus Christus, showing a fly sitting on a fake frame. In 2001, Garnet Hertz produced an art project in which a complete web server was implanted into a dead fly. Tsetse Tsetses are large biting flies from Africa, which live by feeding on the blood of vertebrate animals. Tsetse includes all the species in the genus Glossina, which are generally placed in their own family Glossinidae and which belong to the order Diptera. The genus name is attributed to Wiedemann, who named the type species Glossina longipalpis in 1830. Tsetse has been extensively studied because they are biological vectors of the African trypanosomiases, deadly diseases, which include sleeping sickness in humans and nagana in cattle. Tsetse are crudely similar to other large flies, such as the housefly, Musca domestica, but can be distinguished by four characteristics of their anatomy, two of which are easy to observe. Tsetse folds their wings completely when they are resting so that one wing rests directly on top of the other over their abdomen. Tsetse also has a long proboscis which extends directly forward and is attached by a distinct bulb to the bottom of their head. Tsetse has existed in the modern morphological form for at least 34 million years since fossil tsetse have been recovered from the Florissant Fossil Beds in Colorado. Tsetse biology: The biology of tsetse is relatively well understood (Fig. 45). Tsetse has been extensively studied because of their medical, veterinary, and economic importance, because the flies can be raised in a laboratory, and because the flies are relatively large facilitating their analysis. Entomologists have discovered a great deal about tsetse morphology, anatomy, development and metabolism. Tsetse morphology: Tsetse can be seen as independent individuals in three forms: as third instar larva, as puparia, and as adults. Tsetses first become separate from their mothers during the third larval instar, during which they have the typical appearance of maggots. However, this life stage is short, lasting at most a few hours, and is almost never observed outside of the laboratory. Tsetse next become puparia—small, hard shelled, oblongs with two distinctive, small, dark lobes at one end. Tsetse puparia are under 1.0 centimeters long. Tsetse then emerge as adult flies. Tsetse adults are relatively large flies, with lengths of 0.5 to 1.5 centimeters, and have a recognizable shape or bauplan so they can usually be distinguished without trouble from other flies. Tsetses have large heads, distinctly separated eyes, and unusual antennae. The tsetse thorax is quite large, while the abdomen is wide rather than elongated and shorter than the wings. Four characteristics definitively separate adult tsetse from other kinds of flies: Proboscis — Tsetse have a distinct proboscis, a long thin structure attached to the bottom of the head and pointing forward. Folded wings — When at rest, tsetse fold their wings completely one on top of the other. Hatchet cell — The discal medial ("middle") cell of the wing has a characteristic hatchet shape resembling a meat cleaver or a hatchet. Branched arista hairs — The antennae have arista with hairs, which are themselves branched. Tsetse anatomy: Like all other insects tsetse flies have an adult body comprised of three, visibly distinct, parts: the head, the thorax, and the abdomen. The head has large eyes, distinctly separated on each side, and a distinct, forwardpointing proboscis attached underneath by a large blub. The thorax is large, made of three fused segments. Three pairs of legs are attached to the thorax, as are two wings and two halteres. The abdomen is short but wide and changes dramatically in volume during feeding. The internal anatomy of tsetse is fairly typical of the insects. The crop is large enough to accommodate a huge increase in size during the blood meal since tsetse can take a bloodmeal weighing as much as themselves. The reproductive tract of adult females includes the uterus, which can become large enough to hold the thrid instar larva at the end of each pregnancy. The tsetse life cycle: Tsetse have an unusual life cycle which may be due to the richness of their food source. Female tsetse only fertilize one egg at a time and retain each egg within their uterus to have the offspring develop internally during the first larval stages, a strategy called adenotrophic viviparity. During this time, the female feeds the developing offspring with a milky substance, which is secreted by a modified gland in the uterus. In the third larval stage, the tsetse larva finally leaves the uterus and begins its independent life. However, the newly independent tsetse larva simply crawls into the ground, forms a hard outer shell called the puparial case in which it completes its morphological transformation into an adult fly. This lifestage has a variable duration, generally twenty to thirty days, and the larva must rely on stored resources during this time. The importance of the richness of blood to this development can be seen since all tsetse development prior to the emergence from the puparial case as a full adult occurs without feeding based only on nutritional resources provided by the female parent. The female must obtain enough energy for her needs, for the needs of her developing offspring, and to store the resources, which her offspring will require until it emerges as an adult. Technically these insects undergo the standard development process of insects, which comprises oocyte formation, ovulation and fertilization, development of the egg, five larval stages, a pupal stage, and the emergence and maturation of the adult. Tsetse metabolism: Tsetse metabolism consists of ingesting vertebrate blood, which is called hematophagy, and digesting this blood to obtain energy and biomass. Tsetse have specialized cells that contain bacterial endosymbionts required for survival. An unusual aspect of tsetse metabolism is the particular pathway which tsetse use for flight, which seems to be responsible for the extremely high energy output and elevated flying speeds which tsetse can achieve. Most insects, such as honey bees, consume sugar predominantly for metabolic energy. Tsetse, instead, use a pathway which involves the conversion of the amino acids proline and alanine. The result of this pathway is that tsetse can create large amounts of ATP but can only sustain this metabolic output for short durations. Tsetse therefore fly at very high speeds (they are known to be able to follow a car moving at thirty miles an hour) but can only sustain their flight for short durations of around thirty seconds. General biology: Remarkably the Tsetse Glossina palpalis is also a vector and host of Hepatozoon petti, a parasitic Sporozoa of the nile crocodile. Tsetse systematics: Tsetse include up to thirty-four species and sub-species depending on the particular classification used. Tsetse are sufficiently different in appearance and behavior to have been placed in their own distinct branch of the flies. This placement is controversial. The science of systematics is currently struggling to reconcile the traditional form of biological classification with the modern understanding of genomic evolution and speciation. The controversy surrounding the placement of tsetse is therefore likely to continue into the future. All current classifications place all the tsetse species in a single genus named Glossina. Most classifications place this genus as the sole member of the family Glossinidae. The Glossinidae are generally placed within the infraorder Cyclorrhapha, which includes the housefly and the blowfly due to the similarity of their developmental biology. This infraorder in turn, is part of the sub-order Brachycera, the stubby flies with reduced antenna. Tsetse are evidently part of the order Diptera, the flies. Tsetse as vectors of trypanosomiases: Tsetse are biological vectors of trypanosomes meaning that tsetse, in the process of feeding, acquire and then transmit small, single-celled organisms called trypanosomes from infected vertebrate hosts to uninfected animals. Some tsetse transmitted trypanosome species cause trypanosomiasis, an infectious disease. In humans, tsetse transmitt trypanosomiasis, called sleeping sickness. In animals, tsetse vectored trypanosomiases include nagana, souma, and surra according to the animal infected and the trypanosome species involved, although the usage is not strict and nagana is occasionally used for any form of animal trypanosomiasis. Trypanosomes are animal parasites, specifically protozoa of the genus Trypanosoma. These organisms are approximately the size of red blood cells. Different species of trypanosomes infect different hosts as can be seen in the table attached to this section. Trypanosomes range widely in their effects on the vertebrate hosts. Some species, such as Trypanosoma theileri, do not seem to cause any health problems except perhaps in animals, which are already quite sick. Some strains are much more virulent. Tsetse seem to be unaffected by the infection of trypanosomes but it is entirely possible that the parasites alter tsetse behavior or have other effects which improve the chances of transmission and survival. These trypanosomes have become highly evolved and developed a life cycle, which requires periods in both the vertebrate and tsetse hosts. Tsetse transmit trypanosomes in two ways, mechanical and biological transmission. Mechanical transmission involves the direct transmission of the same individual trypanosomes taken from an infected host into an uninfected host. The name mechanical reflects the similarly of this mode of transmission to the transmission which could be caused mechanically with a syringe. Mechanical transmission requires that tsetse feed on an infected host and acquire trypanosomes in the blood meal, and then, within in a relatively short period, for tsetse to feed on an uninfected host and regurgitate some of the infected blood from the first blood meal into the tissue of the uninfected animal. This type of transmission occurs most frequently when tsetse are interrupted during a bloodmeal and attempt to satiate themselves with another meal. Other flies, such as horse-flies, also can cause mechanical transmission of trypanosomes. Biological transmission requires a period of incubation of the trypanosomes within the tsetse host. The term biological is used because trypanosomes must reproduce through several generations inside the tsetse host during the period of incubation, which requires extreme adaptation of the trypanosomes to their tsetse host. In this mode of transmission, trypanosomes reproduce through several generations, changing in morphology at certain periods. This mode of transmission also includes the sexual phase of the trypanosomes. Tsetse are believed to be more likely to become infected by trypanosomes during their first few bloodmeals. Tsetse infected by trypanosomes are thought to remain infected for the remainder of their lives. Because of the adaptations required for biological transmission, trypanosomes, which are transmitted biologically by tsetse cannot be transmitted in this manner by other insects. The relative importance of these two modes of transmission for the propagation of tsetse-vectored trypanosomiases is not yet well understood. However, since the sexual phase of the trypanosome lifecycle occurs within the tsetse host, biological transmission is a required step in the life cycle of the tsetse vectored trypanosomes. The cycle of biological transmission of trypanosomiasis involves two phases, one inside the tsetse host and the other inside the vertebrate host. Trypanosomes are not passed between a pregnant tsetse and her offspring so all newly emerged tsetse adults are free of infection. An uninfected fly, which feeds upon an infected vertebrate animal may acquire trypanosomes in its proboscis or gut. These trypanosomes, depending on the species, may remain in place, move to a different part of the digestive tract, or migrate through the tsetse body into the salivary glands. When an infected tsetse bites a susceptible host, the fly may regurgitate part of a previous blood meal, which contains trypanosomes or may inject trypanosomes contained within its saliva. It is believed that the inoculation must contain a minimum of 300 to 450 individual trypanosomes to be successful, and may contain up to 40,000 individuals. The trypanosomes are injected into vertebrate muscle tissue but make their way, first into the lymphatic system, then into the bloodstream, and eventually into the brain. The disease causes the swelling of the lymph glands, emaciation of the body, and eventually leads to death. Uninfected tsetse may bite the infected animal prior to its death and acquire the disease, thereby closing the transmission cycle. The tsetse vectored trypanosomiases affect various vertebrate species including humans, antelopes, bovine, cattle, camels, horses, sheep, goats, and pigs. These diseases are caused by several different trypanosome species, which may also survive in wild animals such as crocodiles and monitor lizards. The diseases have different distributions across the african continent and are therefore transmitted by different species of tsetse. Tsetse vectored human trypanosomiases: Human African trypanosomiasis, also called sleeping sickness, is caused by trypanosomes of the Trypanosoma brucei species. This disease is invariably fatal unless treated but can almost always be cured with current medicines, if the disease is caught early enough. Sleeping sickness begins with a tsetse bite leading to an inoculation in the sub-cutaneous tissue. The infection moves into the lymphatic system leading to a characteristic swelling of the lymph glands, which is called Winterbottoms's sign. The infection progresses into the blood stream and eventually crosses into the central nervous system and invades the brain leading to extreme lethargy and eventually to death. The page on human sleeping sickness has more extensive information about this disease. The Trypanosoma brucei species, which causes the disease, has often been subdivided into three sub-genera, which were identified based either on the vertebrate hosts, which the strain could infect or on the virulence of the disease in humans. The trypanosomes infectious to animals and not to humans were named Trypanosoma brucei brucei. The strains which infected humans were divided into two sub-species based on their different virulences: Trypanosoma brucei gambiense was thought to have a slower onset and Trypanosoma brucei rhodesiense refers to strains with a more rapid, virulent onset. This characterization has always been problematic but was the best that could be done given the knowledge of the time and the tools available for identification. A recent molecular study using restriction fragment length polymorphism analysis suggests that the three sub-genera are polyphyletic, so the elucidation of the strains of T. brucei infective to humans will require a more complex explanation. Other forms of human trypanosomiasis also exist but are not transmitted by tsetse. The most notable is American trypanosomiasis, known as Chagas disease, which occurs in South America, caused by Trypanosoma cruzi, and transmitted by certain species of the reduviidae, members of the true bugs. Tsetse vectored animal trypanosomiases: Animal trypanosomiasis, also called nagana when it occurs in bovine cattle or horses or sura when it occurs in domestic pigs, is caused by several trypanosome species. These diseases reduce the growth rate, milk productivity, and strength of farm animals, generally leading to the eventual death of the infected animals. Certain species of cattle are called trypanotolerant because they can survive and grow even when infected with trypanosomes although they also have lower productivity rates when infected. The course of the disease in animals is similar to the course of sleeping sickness in humans. Trypanosoma congolense and Trypanosoma vivax are the two most important species infecting bovine cattle in sub-saharan Africa. Trypanosoma simiae causes a virulent disease in pigs. Other forms of animal trypanosomiasis are also known from other areas of the globe, caused by different species of trypanosomes and transmitted without the intervention of tsetse. Tsestse control: Tsetse control has been undertaken in order to reduce the incidence of the diseases, which the flies transmit. Two alternative strategies have been used in the attempts to reduce the African trypanosomiases. One tactic is primarily medical or veterinary and targets the disease directly using monitoring, prophylaxis, treatment, and surveillance to reduce the number of organisms, which carry the disease. The second strategy is generally entomological and intends to disrupt the cycle of transmission by reducing the number of flies. The idea of tsetse control implies a change in the relationship between people and these insects. Prior to the twentieth century, people in Africa had largely adapted to the presence of tsetse. Human settlement patterns and agricultural practices had adapted to the presence of the fly. For example, in Ethiopia draft powered framing was restricted to the highland areas where the flies were absent whereas lowland areas where tsetse are present were more sparsely populated by people living a nomadic, less agriculturally intensive lifestyle. Tsetse control is a response to changing conditions. Tsetse control has been proposed as a way of reducing the incidence of the disease in the populations living in tsetse regions, of allowing the expansion of human settlement and agriculture into new areas, and of helping people previously relocated either in forced transfers or due to migration. Tsetse control efforts have been undertaken throughout the African continent but long-term, sustainable control has rarely been achieved. Tsetse control efforts invariably are tied to the complex problems of poverty, heath, politics, and violence, which have proved such a disaster for the African people. The reduction of fly numbers has generally been attempted with two different aims, either eradication which intends to completely eliminate tsetse from the area or control, which aims simply to reduce the numbers. Eradication is an idea which has often been imagined, has repeatedly been attempted, and is still proposed but many reasons suggest that control is a safer, cheaper, more realistic, and sustainable approach. Eradication refers to the successful killing of every tsetse either in a region or, under more grandiose proposals, from the entire African continent. Local eradication efforts have repeatedly been undertaken and have achieved temporary success only to fail in the long term because tsetse were able to re-invade (Sansibar). All of the economic, ecological, political, and environmental justifications for eradication have been called into question. The economic justification for eradication offsets the immense costs of the eradication campaign against the medical and veterinary benefits, which are considered to accrue in perpetuity. Etymology: The word “tsetse” comes from Tswana, a language of southern Africa, and, in that language, the word means fly. Because of this meaning, the phrase “tsetse fly” is redundant. This is an example of "incomplete incorporation". Recently “tsetse” without the “fly” has become more common in English, particularly in the scientific and development communities. Because most people outside of Africa learn the word later in life through formal means, e.g. books and documentaries, the use of tsetse alone is likely to become dominant. The pronunuciation of the word differs in different regions. Many African languages have an ejective ts sound and so a common pronunciation of the word involves two identical syllables both having this ts sound and a shorter sound of the vowel, as tseh-ts-eh. The British pronunciation of the word uses two different sounds for the two different syllables, generally tee-tsee. In Zimbabwe, it is generally pronounced tseh-tsee. Oestridae Oestridae (also called botfly or bot fly) is a family of Oestroidea. It is one of several families of hairy flies whose larvae live as parasites within the bodies of mammals, such as the Desert Woodrat. Life cycle: Adult botflies deposit eggs on a host body: common houseflies for example. Eggs are deposited on animal skin by their host: the body heat of the animal induces hatching upon contact. Some forms of botfly also reside in the digestive tract when consumed by a licking action. Myiasis: larvae burrow into the skin (or tissue lining) of the new host animal. Mature larvae drop from the host and complete the pupae stage in soil. Remedies: Immediate contact with larvae can be remedied with alcohol. If one is afflicted with bot flies, one "cure" (which is really more of a folk remedy) is to put meat over the affected area while the flies are in their larval stage, thereby cutting off the parasites' air supply. The grubs should then burrow through the meat to gain access to oxygen, at which point the meat may be removed with the larvae trapped inside. The botfly maggot cannot be removed easily whilst alive due to the spines that run along its body. One medical treatment is to suffocate the grub by sealing off the air hole found in the surrounding blister. This can be done with petroleum jelly or a similar substance. This forces the grub to expose itself, making it easier to remove. Deer Botfly The deer botfly was reported for many years to be the fastest of all flying insects, cited by the New York Times and Guinness Book of World Records as traveling at speeds of over 800 miles per hour. The source of this remarkable claim was an article by entomologist Charles H. T. Townsend in the 1927 Journal of the New York Entomological Society, wherein Townsend claimed to have estimated a speed of 400 yards per second while observing botflies at 12,000 feet in New Mexico. In 1938, Nobel laureate chemist Irving Langmuir examined the claim in detail and refuted the estimate. Among his specific criticisms were: To maintain a velocity of 800 miles per hour, the 0.3-gram fly would have had to consume more than 150% of its body weight in food every second; The fly would have produced an audible sonic boom; The supersonic fly would have been invisible to the naked eye; The impact trauma of such a fly colliding with a human body would resemble that of a gunshot wound. Using the original report as a basis, Langmuir estimated the deer botfly's true speed at 25 miles per hour.