Organic Objectives

Students will know…EVERYTHING IN THE PACKET
the bond angle of H-C-H bond.
the bonding capacity of C, H, O, Cl, Br, and I.
that the parent chain can be bent.
that hydrocarbons are good fuels.
the names of side groups: methyl –CH
3
ethyl –C
2
H
5
bromo –Br iodo –I chloro –Cl
to start numbering carbons of a parent chain where the side group is closest .
that “n” means “normal” (straight chain.) For example, n-pentane.
“I” means iso, and “t” means tertiary (neo is also used) and how to draw and name these
that tetramethyl means 4 methyl groups, trimethyl means 3 groups, dimethyl means 2 groups, etc.
that hydrocarbons with: all single bonds are called alkanes one double bond are called alkenes end with “ane” end with “ene” follow the formula
C n
H
2n+2 follow the formula C n
H
2n one triple bond are called alkynes end with “yne” follow the formula
C n
H
2n-2
that “cyclo-“ means the molecule contains a ring.
that “saturated” means “saturated with hydrogens” and describes alkanes.
that alkenes, alkynes, and cyclic hydrocarbons are all “unsaturated.”
that unsaturated fats are better for you (the double bond makes them easier to digest and less likely to coat the inside of your arteries.)
that various types of compounds (alcohols, ethers, etc.) have atoms in common (called functional groups).
that “R” stands for the “Rest of the molecule” (methyl, ethyl, etc.) [Officially,
“R” = “Residue”.]
examples of these molecules in daily life. (methyl alcohol, ethyl alcohol, denatured alcohol, rubbing alcohol, formaldehyde, acetone, diethylether, acetic acid, citric acid, salicylic acid, methylsalicylate, vitamins)
Acid + Alcohol Ester + H
2
O
Amino Acid + Amino Acid Protein + H
2
O
that one way molecules can join, a reaction where H
2
O is formed, is called a “condensation reaction.”
that a polymer (“poly” means many, “mer” means parts) consists of many repeating parts.
a second way molecules can join, in which a double bond opens to form two new bonds, is called an “addition reaction.”
Organic Chemistry: Honors
Students will be able to…
give examples of hydrocarbons.
give examples of condensed formulas, skeletal formulas and structural formulas.
list prefixes 1 – 10 (meth-, eth-, prop-, but-, etc.)
build models of molecules.
state the # of atoms and # of elements in a molecule.
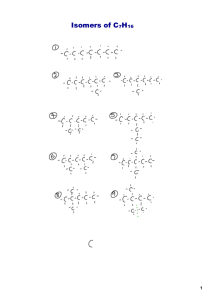
draw isomers of molecules and recognize isomers.
write the formula of a hydrocarbon, given its name and vice versa.
give household examples of hydrocarbons (methane, ethane, propane, butane, octane).
identify the “parent chain” looking at a structural formula
give examples of substituted hydrocarbons.
draw and name isomers of substituted hydrocarbons.
build models using single, double and triple bonds and describe which rotate.
demonstrate cis- and trans- isomerism, example: dichloroethene, C
2
H
2
Cl
2
.
recognize whether the molecule is an alkane, alkene, or alkyne given the formula.
name a molecule, given the structural formula.
write the correct structural formula, given the name.
circle the atoms that make up the functional group, given the structural formula of a molecule.
Draw the mechanism for making a ketone from an alkyne
Name alkanes, alkenes, alkynes, haloalkanes, cyclo’s, alcohols, aldehydes, ketones, carboxylic acids.
Know how to map and show the exact chemistry, including side products for multistep synthesis.
Identify and draw primary, secondary, and tertiary alcohols.
Know which of the alcohols are reactive and which are not.
Identify and draw and name iso and tert (t) (neo) configurations.
Know what an “alkyl” group is
Know the structure of acetylene, phenol, acetic acid
Know the ortho, para, meta positions on phenol rings.
Know that the OH group as a functional group is alcholo/alkonols and as a substituent is hydroxy.
Know that the =O is a ketone but is called oxo as a substituent group.
Know Markovnikov rule.
Know the mechanism for adding 2 H
2
O to an alkyne to make a ketone or alkanal.
SEE OTHER SIDE
Naming Helps:
The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing.
Dimethylpentyl (as complete single substituent) is alphabetized under
"d", thus 7-(1,2-
Dimethylpentyl)-5ethyltridecane
If several substituents are present on the ring, they are listed in alphabetical order. Location numbers are assigned to the substituents so that one of them is at carbon #1 and the other locations have the lowest possible numbers, counting in either a clockwise or counterclockwise direction.
If he ene suffix (ending) indicates an alkene or cycloalkane then the longest chain chosen for the root name must include both carbon atoms of the double bond.
Double bonds precede triple bonds in the IUPAC name, but the chain is numbered from the end nearest a multiple bond, regardless of its nature.
Iso and tert are used when alphabetizing. ( We will do it this way but I have found conflicting info.)
CH3-CH2-CH2-CH=CH-CH3 hex-2-ene (Preferred)
(not 2-hexene)
CH2=CH-CH2-CH2-OH but-3-en-1-ol (PIN)
Hyphenated prefixes: sec- and tert- are not used in alphabetizing
but iso is. Thus isopropyl and isobutyl are listed alphabetically under i but sec-butyl and tert-butyl are listed under b.
Recognize and draw the structures below alcohol aldehyde carboxylic acid
–
ester ketone amine ether (bunny) amide
Aromatic Rings Haloalkanes
R
X
state natural examples of polymers such as proteins (many amino acids), carbohydrates
(many sugar units), and DNA (many nucleotide bases).
state man-made polymers like polyester (many ester linkages), and polyethylene, polystyrene, and Teflon.
draw the “repeat unit” with the double bond open and copy the repeat unit to draw the polymer, given a monomer (like ethane, aka. ethylene),
give examples of common substances made from addition polymers.