Chapter 11 & 12 Review
advertisement

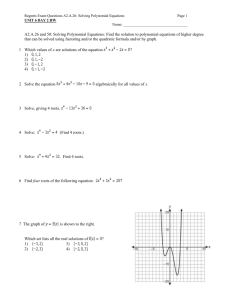
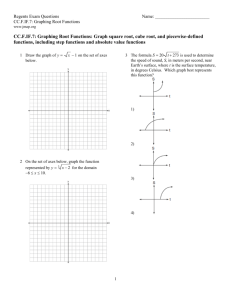
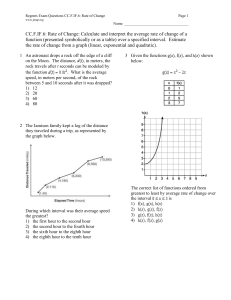
Chapter 11 & 12 Review Remember to study actively, memorizing the answers will not help you TRUE/FALSE 1. All carbohydrates, proteins, and fats (lipids) are organic compounds. ANS: T REF: K/U OBJ: 11.1 LOC: HE1.01 2. The study of hydrocarbons is the study of a class of organic compounds that contain mostly carbon and hydrogen but may also contain other elements such as oxygen and nitrogen. ANS: F REF: K/U OBJ: 11.1 LOC: HE1.01 3. In the early 19th century, most chemists believed that organic compounds could not be produced in the laboratory setting. ANS: T REF: K/U OBJ: 11.1 LOC: HE1.01 4. The production of the first organic compounds in the lab was due to chemists being able to duplicate the "vital force" present in living organisms. ANS: F REF: K/U OBJ: 11.1 LOC: HE1.01 5. Based on their physical properties, hydrocarbons are all nonpolar substances. ANS: T REF: K/U OBJ: 11.1 LOC: HE1.03 6. All organic compounds can be classified as though they were hydrocarbons that were changed by addition, subsitution, and atom rearrangement. ANS: T REF: K/U OBJ: 11.1 LOC: HE1.03 7. This is one way to represent the structure of propane. ANS: T REF: I OBJ: 11.1 LOC: HE2.02 8. Aliphatic compounds all contain the ring structure called benzene. ANS: F REF: K/U OBJ: 11.1 LOC: HE1.02 9. The homologous series of hydrocarbons that contain only single carbon to carbon bonds with an open chain are called alkanes. ANS: T REF: K/U OBJ: 11.1 LOC: HE1.02 10. Petroleum is the fuel that is used in automobiles. ANS: F REF: K/U OBJ: 11.2 LOC: HE1.01 11. Natural gas is the compound that emerges as gases from drilled wells and contain only those compounds with low molar masses. ANS: T REF: K/U OBJ: 11.2 LOC: HE1.01 12. In fractional distillation, the compounds with the lowest boiling points condense in the lower parts of the tower. ANS: F REF: K/U OBJ: 11.2 LOC: HE1.03 13. The process of cracking causes a larger hydrocarbon molecule to break into two smaller hydrocarbon molecules. ANS: T REF: K/U OBJ: 11.2 LOC: HE3.01 14. Reforming is the rearrangement of the carbon and hydrogen atoms in a hydrocarbon. ANS: F REF: K/U OBJ: 11.2 LOC: HE3.01 15. Oxygen is not always needed for combustion reactions to occur. ANS: F REF: I OBJ: 11.3 LOC: HE2.05 16. When dealing with the incomplete combustion of hydrocarbons, carbon dioxide, carbon monoxide, and carbon can all be products of the reaction. ANS: T REF: I OBJ: 11.3 LOC: HE2.05 17. Isomers are chemicals with the same properties but with different molecular formulas. ANS: F REF: K/U OBJ: 11.4 LOC: HE1.02 18. The naming of branched alkanes can follow two different systems—either the branches are named in alphabetical order or in order of complexity (number of carbons in the branch). ANS: T REF: I OBJ: 11.4 LOC: HE2.02 19. Alkanes are considered to be saturated since they contain the maximum number of hydrogen atoms attached to the carbon backbone. ANS: T REF: K/U OBJ: 11.4 LOC: HE2.01 20. The following molecule can correctly be named 1-butene or 3-butene. ANS: F REF: I OBJ: 11.5 LOC: HE2.02 21. In geometric isomers the term "cis-" indicates that the two main groups on the molecule are "across" the double bond, that is on opposite sides of the molecule. ANS: F REF: I OBJ: 11.5 LOC: HE2.03 22. All polymers are synthetic, that is they are man-made products. ANS: F REF: I OBJ: 11.5 LOC: HE3.02 1.An energy resource is a natural substance or process that provides a useful form of energy. ANS: T REF: K/U OBJ: 12.1 LOC: HE1.06 2. Most familiar forms of energy are eventually converted to kinetic energy. ANS: F REF: K/U OBJ: 12.1 LOC: HE1.05 3. The specific heat capacity is the quantity of heat required to change the temperature of a unit mass of a substance by one degree Celsius. ANS: T REF: K/U OBJ: 12.1 LOC: HE1.05 4. The larger the specific heat capacity of a particular substance, the larger the temperature change that substance will experience from a given amount of energy. ANS: F REF: K/U OBJ: 12.1 LOC: HE1.05 5. The burning of hydrocarbons is an example of an endothermic reaction. ANS: F REF: K/U OBJ: 12.1 LOC: HE1.06 6. Exothermic changes involve the release of energy to the surroundings. ANS: T REF: K/U OBJ: 12.1 LOC: HE1.06 7. Temperature, theoretically, is a measure of the average kinetic energy of the molecules in a substance. ANS: T REF: K/U OBJ: 12.1 LOC: HE1.05 8. According to one of the laws of thermodynamics, heat always transfers from a cooler object to a warmer object. ANS: F REF: K/U OBJ: 12.2 LOC: HE1.05 9. Inside a calorimeter, the amount of energy gained by the surroundings (the water) must equal the amount of energy released by the reaction in the calorimeter. ANS: T REF: I OBJ: 12.2 LOC: HE2.07 10. It can be assumed that all bomb calorimeters have the same heat capacity. ANS: F REF: I OBJ: 12.3 LOC: HE2.07 11. The molar heat of reaction refers to the amount of heat needed to increase the temperature of one mole of a substance by one degree Celsius. ANS: F REF: K/U OBJ: 12.3 LOC: HE1.05 12. The amount of energy required to break a chemical bond is referred to as bond energy. ANS: T REF: K/U OBJ: 12.3 LOC: HE1.04 13. Breaking bonds requires energy, while forming bonds releases energy. ANS: T REF: K/U OBJ: 12.3 LOC: HE1.04 14. In an endothermic reaction, the bond energy of the products is greater than the bond energy of the reactants. ANS: F REF: K/U OBJ: 12.3 LOC: HE1.04 15. In an exothermic reaction, more energy is released in forming new bonds than is absorbed in breaking the bonds in the reactants. ANS: T REF: K/U OBJ: 12.3 LOC: HE1.04 16. Canada is the world's second largest per capita consumers of energy. ANS: T REF: MC OBJ: 12.4 LOC: HE3.02 17. Most of our current fossil fuel production is used to provide petrochemicals for manufacturing plastics. ANS: F REF: MC OBJ: 12.4 LOC: HE3.02 18. Although 95% of our fossil fuel production is burned for energy, the jobs created from the 5% used for petrochemicals is much greater. ANS: T REF: I OBJ: 12.4 LOC: HE3.02 1.DNA, RNA, chromosomes, and genes are composed of ____________________ compounds. ANS: organic LOC: HE1.01 REF: K/U OBJ: 11.1 2. Polyethylene, polypropylene, nylon, and polyester are organic ____________________. ANS: polymers REF: K/U OBJ: 11.1 LOC: HE1.01 3. A theory called ____________________ proposed that the laws of nature were somehow different for living and nonliving systems and that organic compounds could not be synthesized outside of living organisms. ANS: vitalism LOC: HE1.01 REF: K/U OBJ: 11.1 4. The theory of vitalism was shown to be unacceptable when Wohler was able to synthesize ____________________ in a laboratory setting. ANS: urea LOC: HE1.01 REF: K/U OBJ: 11.1 5. The physical properties of hydrocarbons form clear trends largely explained by ____________________. ANS: London forces dispersion forces REF: K/U OBJ: 11.1 LOC: HE1.03 6. Coal, crude oil, oil sands, heavy oil, and natural gas are the ____________________ sources of fuels that power our society. ANS: nonrenewable LOC: HE3.02 REF: I OBJ: 11.1 7. Coal, crude oil, oil sands, and heavy oil are classified as ____________________ because of the way they are formed. ANS: fossil fuels LOC: HE1.01 REF: K/U OBJ: 11.1 8. ____________________ is the technology that includes separating complex mixtures into more simple, purified components. ANS: Refining LOC: HE3.01 REF: I OBJ: 11.1 9. Organic compounds that are classified as aromatic are based on a ring-type structure called ____________________. ANS: benzene ( LOC: HE1.02 ) REF: K/U OBJ: 11.1 10. ____________________ is a complex mixture consisting primarily of hydrocarbons. ANS: Crude oil LOC: HE1.01 REF: K/U OBJ: 11.2 11. The differences in boiling points or the compounds making up petroleum allow us to physically separate these componds in the process called _________________________. ANS: fractional distillation LOC: HE3.01 REF: I OBJ: 11.2 12. The term ____________________ refers to the trapping of thermal energy in the atmosphere. ANS: greenhouse effect LOC: HE3.02 REF: I OBJ: 11.2 13. Many reactions involving alkanes are relatively slow at SATP because these molecules are considered to be ____________________. ANS: saturated LOC: HE2.01 REF: I OBJ: 11.3 14. _________________________ are hydrocarbons with the same molecular formula, but with different structural diagrams. ANS: Structural isomers LOC: HE2.03 REF: C OBJ: 11.3 15. Organic compounds with double and triple carbon-to-carbon bonds are said to be ____________________ because fewer atoms are attached to the carbon framework than if the bonds were single. ANS: unsaturated REF: I OBJ: 11.5 LOC: HE2.01 1.Coal, oil, and natural gas are natural resources whose ____________________ energy is converted into heat and other forms of energy. ANS: chemical LOC: HE3.02 REF: K/U OBJ: 12.1 2. ____________________ energy and ____________________ energy are often too variable or produce too little energy. They are, therefore, used to a limited extent. ANS: Solar, geothermal LOC: HE3.02 REF: K/U OBJ: 12.1 3. ____________________ is the energy that is transferred between substances. ANS: Heat LOC: HE1.05 REF: K/U OBJ: 12.1 4. The temperature change of a substance, , varies ____________________ with the quantity of heat, q, flowing into or out of the substance. ANS: directly LOC: HE1.05 REF: K/U OBJ: 12.1 5. Each substance has a _________________________ that indicates the quantity of energy required to produce a given temperature change per unit mass of the substance. ANS: specific heat capacity LOC: HE1.05 REF: K/U OBJ: 12.1 6. Compared to most other substances, water has a relatively ____________________ specific heat capacity. ANS: high LOC: HE1.05 REF: K/U OBJ: 12.1 7. A reaction in which energy is ____________________ to its surroundings is classified as exothermic. ANS: released LOC: HE1.06 REF: K/U OBJ: 12.1 8. A reaction that absorbs energy from its surroundings is classified as ____________________. ANS: endothermic LOC: HE1.06 REF: K/U OBJ: 12.1 9. ____________________ is the energy absorbed or released by a system per degree Celsius and is represented by the symbol "c". ANS: Heat capacity LOC: HE2.07 REF: I OBJ: 12.2 10. A chemical equation that includes the heat transferred during the reaction is called a _________________________. ANS: thermochemical equation LOC: HE2.06 REF: I OBJ: 12.3 11. The conversion factor between a specific heat of reaction and a molar heat of reaction is the ____________________ of the substance. ANS: molar mass LOC: HE2.06 REF: I OBJ: 12.3 12. ____________________ is the energy required to break a chemical bond. ANS: Bond energy LOC: HE1.04 REF: K/U OBJ: 12.3 13. Electrolysis of water is the simplest way to decompose water into its constituent elements. Electricity must be continually supplied to ____________________ the bonds between hydrogen and oxygen. ANS: break LOC: HE1.04 REF: K/U OBJ: 12.3 14. The quantity of heat transferred in a chemical reaction is called the ____________________. ANS: heat of reaction LOC: HE2.07 REF: I OBJ: 12.3 15. _________________________ is equal to the mass of the substance multiplied by the specific heat capacity of the substance multiplied by the change in temperature of the substance. ANS: The quantity of heat (q). LOC: HE2.07 REF: I OBJ: 12.2 Essay Questions 1.DNA, RNA, chromosomes, and genes are composed of ____________________ compounds. ANS: organic LOC: HE1.01 REF: K/U OBJ: 11.1 2. Polyethylene, polypropylene, nylon, and polyester are organic ____________________. ANS: polymers LOC: HE1.01 REF: K/U OBJ: 11.1 3. A theory called ____________________ proposed that the laws of nature were somehow different for living and nonliving systems and that organic compounds could not be synthesized outside of living organisms. ANS: vitalism LOC: HE1.01 REF: K/U OBJ: 11.1 4. The theory of vitalism was shown to be unacceptable when Wohler was able to synthesize ____________________ in a laboratory setting. ANS: urea LOC: HE1.01 REF: K/U OBJ: 11.1 5. The physical properties of hydrocarbons form clear trends largely explained by ____________________. ANS: London forces dispersion forces REF: K/U OBJ: 11.1 LOC: HE1.03 6. Coal, crude oil, oil sands, heavy oil, and natural gas are the ____________________ sources of fuels that power our society. ANS: nonrenewable LOC: HE3.02 REF: I OBJ: 11.1 7. Coal, crude oil, oil sands, and heavy oil are classified as ____________________ because of the way they are formed. ANS: fossil fuels LOC: HE1.01 REF: K/U OBJ: 11.1 8. ____________________ is the technology that includes separating complex mixtures into more simple, purified components. ANS: Refining LOC: HE3.01 REF: I OBJ: 11.1 9. Organic compounds that are classified as aromatic are based on a ring-type structure called ____________________. ANS: benzene ( LOC: HE1.02 ) REF: K/U OBJ: 11.1 10. ____________________ is a complex mixture consisting primarily of hydrocarbons. ANS: Crude oil LOC: HE1.01 REF: K/U OBJ: 11.2 11. The differences in boiling points or the compounds making up petroleum allow us to physically separate these componds in the process called _________________________. ANS: fractional distillation LOC: HE3.01 REF: I OBJ: 11.2 12. The term ____________________ refers to the trapping of thermal energy in the atmosphere. ANS: greenhouse effect LOC: HE3.02 REF: I OBJ: 11.2 13. Many reactions involving alkanes are relatively slow at SATP because these molecules are considered to be ____________________. ANS: saturated LOC: HE2.01 REF: I OBJ: 11.3 14. _________________________ are hydrocarbons with the same molecular formula, but with different structural diagrams. ANS: Structural isomers LOC: HE2.03 REF: C OBJ: 11.3 15. Organic compounds with double and triple carbon-to-carbon bonds are said to be ____________________ because fewer atoms are attached to the carbon framework than if the bonds were single. ANS: unsaturated LOC: HE2.01 REF: I OBJ: 11.5 Essay Questions 1.Describe and give examples of the different ways in which hydrocarbons can be expressed. 2.Explain, using examples, why the physical properties of alkenes are similar to alkanes, but the chemical properties are quite different. 3."Of the more than 10 million compounds known, at least 90% are molecular compounds of the element carbon." Explain. 4.Explain the process of refining petroleum from the point of extracting it from an underground deposit through the process of fractional distillation into its many useful components. Indicate some of the compounds that are refined in this way. 5.If Earth's supply of petroleum was to suddenly disappear, how would your life change? Explain by using examples of consumer goods that would no longer be available to you. 6.The term "global warming" has become a well-recognized term in the past few years. What is global warming and what are the most likely causes of it? What are the predicted effects if this trend continues? 2.Canada's petrochemical industry provides not only heat and energy for Canadians but also numerous jobs. Use an example of a petrochemical such as ethylene and discuss the number of jobs that are created by the petrochemical industry. 1. In 1997, Canada was ranked as the second largest consumer of energy per capita. It was outdone only by the United States. Discuss reasons why Canada may need such a large consumption of energy and suggest ways in which some of this energy can be conserved.