Density of Water Vapor Pressure of Water
advertisement

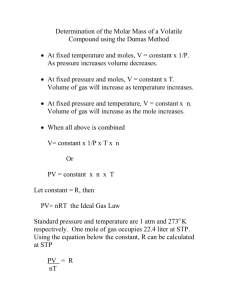
Name: ______________________________________________________ Date: _________ Period: ____ Molar Mass of a Volatile Liquid Background The molar masses of compounds are used daily in the chemistry profession. The molar mass is defined as the mass, in grams, of 1 mole of any element or compound. How is molar mass determined and how is the molar mass of an unknown found? In this experiment, the molar mass of a volatile liquid will be determined based on measurements of vapor density. The ideal gas law relates the four measurable properties of a gas (P, V, n, T). In this experiment, the ideal gas law will be used to determine the molar mass of gases and nonvolatile liquids. PV = nRT Equation 1 The number of moles (n) of any pure substance is equal to the mass of the substance divided by the molar mass of the substance. n = mass/molar mass Equation 2 Substituting for n in Equation 1 and then rearranging produce the equation for the molar mass of a gas. molar mass = mass (g) x RT PxV Equation 3 The molar mass of an unknown volatile liquid with a boiling point well below the boiling point of water will be determined. A small sample of the liquid will be placed in a tared 125 mL erlynmeyer flask and the flask will be heated in boiling water to vaporize the liquid. The air and excess vapor escape, leaving the flask filled only with the volatile liquid vapor at atmospheric pressure and at the temperature of boiling water. The flask is removed and cooled to condense the vapor. Once cooled, the flask is weighed. By massing the same flask filled with distilled water, the volume of the flask is calculated. The molar mass of the volatile liquid is then determined from Equation 3 using the mass of the condensed vapor, the volume of the flask, the atmospheric pressure, and the temperature of the boiling water. Pre-Lab Questions Read the entire procedure carefully before beginning the exercise. 1) A determination of the molar mass of methyl alcohol (CH3OH) yielded the following data: Temperature of boiling water bath Barometric pressure Temperature of room temp. water bath Density of water at room temp. Mass of empty flask Mass of flask and condensed methyl alcohol Mass of flask and water Mass of condensed methyl alcohol Mass of water in filled pipet Volume of pipet Molar mass of methyl alcohol (experimental) Molar mass of methyl alcohol (theoretical) 99.5oC 738 mm Hg 24.0oC 0.9973 g/mL Trial 1 1.557 g 1.571 g 16.001 g Using the data, fill in the rest of the table. The volume of the flask is equal to the volume of water in the flask. Use the relationship of mass and density to determine this volume. Once the volume of the pipet is determined, equation 3 in the Background section can be used to calculate the molar mass of methyl alcohol. Compare this value to the actual molar mass of methyl alcohol. 2) In this lab, you will be asked to identify an unknown volatile liquid. The liquid must be one of the following compounds. Determine the molar mass of each of the possible unknown liquids. Compound acetone methanol (CH3OH) ethanol (C2H5OH) isopropanol (C3H7OH) Molar Mass (g/mol) Materials Chemicals: Unknown volatile liquid Deionized water Equipment 125 mL erlynmeyer flask Rubber stopper to fit flask Eyedropper or glass tubing to fit in rubber stopper Thermometer Milligram balance Barometer Boiling chips 1 L beaker Hot plate Ring stand Clamp Procedure 1) Place a 1 L beaker on the hot plate and add about 500 mL water to the beaker, along with several boiling chips. Turn on the hot plate to boil the water. 2) Obtain a 125 mL erlynmeyer flask and rubber stopper with glass tubing. Mass the flask and stopper apparatus to the nearest 0.001 g. Record the mass in Data Table 1. 3) Place 5.00 mL of an unknown volatile liquid in the bottom of the erlynmeyer flask. 4) Place the rubber stopper in the erlynmeyer flask and mark the bottom of the stopper. 5) Clamp the flask as high up on the neck as possible and slowly lower the flask into the boiling water. Lower the clamp until it touches the lip of the beaker. Clamp in place. (Do not inhale the vapor from the flask.) 6) Continue heating about two minutes after all the liquid in the flask has evaporated. The vapor will force the air from the flask, which will finally contain only the vapor. 7) During this period, take the temperature of the water, placing the bulb of the thermometer on a level with the middle of the flask. 8) Take the flask out of the water and, while it is still warm, wipe it and the outside surface of the foil dry. Allow the flask to cool for 10 minutes with the rubber stopper still in place. 9) Weigh the assembly and its contents. 10) Record the atmospheric pressure. The total pressure of the gases in the flask is equal to this. 11) Remove the rubber stopper and fill the flask to the line where you marked the bottom of the stopper with water, making certain there are no bubbles of air trapped in it. 12) Dry the outside of the flask and then mass the flask and water. Record the mass in the data table. 13) If time permits, repeat the experiment. Data Analysis 1) Determine the mass of condensed, volatile vapor. Enter this value in the data table. 2) Using the information provided, determine the density of water placed in the flask. Use this value and the mass of water in the flask to calculate the volume of the flask. 3) Determine the mass of the condensed volatile liquid. 4) Identify the unknown volatile liquid. 5) Calculate the percent error of your calculated molar mass. Conclusion 1) Volatile liquids with lower boiling points often give better results than those with higher boiling points. Suggest a reason for this. 2) What effect would vapor condensing in the neck of the flask have on the reported molar mass? How large an error might this introduce? 3) Did all of the vapor condense into the liquid? If not, how would this affect the calculated molar mass? 4) Why is not necessary to be precise when the liquid is measured out into the test tube? Data Tables Table 1. Temperature of boiling water Barometric pressure Temperature of water in flask when measuring volume Density of water in the flask Mass of empty flask with rubber stopper Mass of flask and water Mass of water in flask Volume of flask Table 2. Mass of flask and condensed liquid Mass of condensed liquid Molar mass of unknown liquid Density of Water Vapor Pressure of Water Temperature Density Vapor Pressure oC g/mL torr 15 0.9991026 12.79 16 0.9989460 13.64 17 0.9987779 14.54 18 0.9985986 15.49 19 0.9984082 16.49 20 0.9982071 17.55 21 0.9979955 18.66 22 0.9977735 19.84 23 0.9975415 21.09 24 0.9972995 22.40 25 0.9970479 23.78 26 0.9967867 25.24 27 0.9965162 26.77 28 0.9962365 28.38 29 0.9959478 30.08 30 0.9956502 31.86