File - Wk 1-2
advertisement
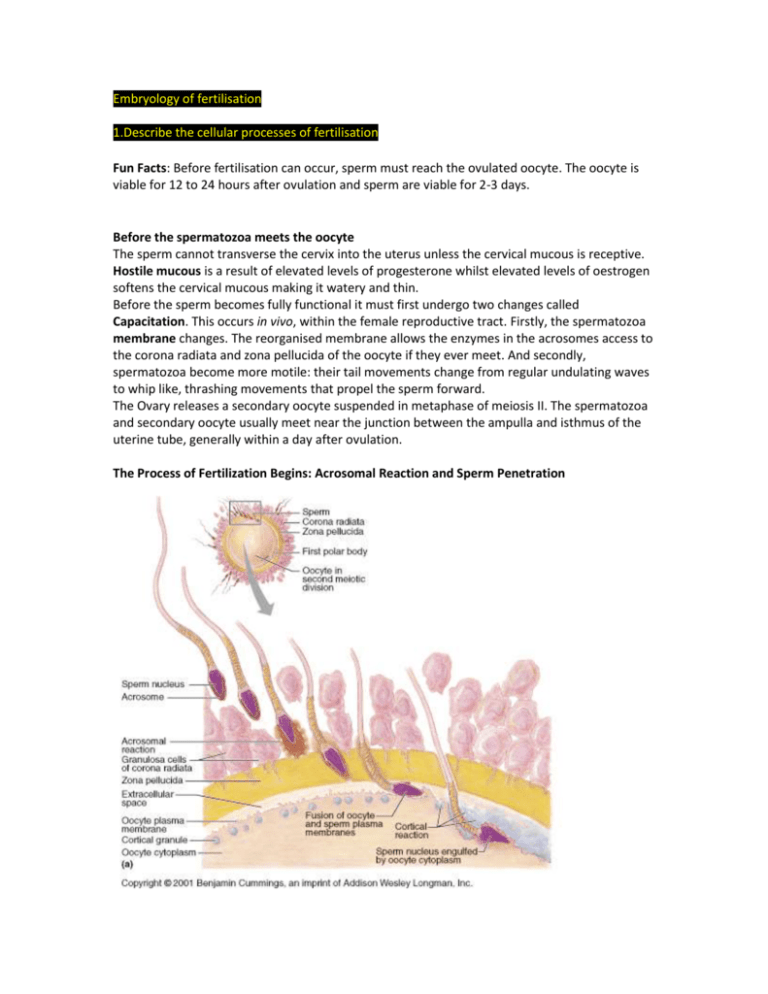
Embryology of fertilisation 1.Describe the cellular processes of fertilisation Fun Facts: Before fertilisation can occur, sperm must reach the ovulated oocyte. The oocyte is viable for 12 to 24 hours after ovulation and sperm are viable for 2-3 days. Before the spermatozoa meets the oocyte The sperm cannot transverse the cervix into the uterus unless the cervical mucous is receptive. Hostile mucous is a result of elevated levels of progesterone whilst elevated levels of oestrogen softens the cervical mucous making it watery and thin. Before the sperm becomes fully functional it must first undergo two changes called Capacitation. This occurs in vivo, within the female reproductive tract. Firstly, the spermatozoa membrane changes. The reorganised membrane allows the enzymes in the acrosomes access to the corona radiata and zona pellucida of the oocyte if they ever meet. And secondly, spermatozoa become more motile: their tail movements change from regular undulating waves to whip like, thrashing movements that propel the sperm forward. The Ovary releases a secondary oocyte suspended in metaphase of meiosis II. The spermatozoa and secondary oocyte usually meet near the junction between the ampulla and isthmus of the uterine tube, generally within a day after ovulation. The Process of Fertilization Begins: Acrosomal Reaction and Sperm Penetration The ovulated oocyte is encapsulated by the corona radiata and the deeper zona pellucida, a transparent layer of glycoprotein-rich extracellular matrix secreted by the oocyte, and both must be breached before the oocyte can be penetrated. Once a sperm gets to the immediate vicinity of the oocyte, it penetrates the corona radiata, assisted by a cell-surface hyaluronidase that breaks down the adjacent bonds between the granulosa cells in the corona radiata, causing them to fall away from the oocyte. After breaching the corona, the sperm head binds to the ZP3 glycoprotein of the zona pellucida, which functions as a sperm receptor and helps trigger the acrosomal reaction. This step causes the rupture of the acrosomal cap. The acrosomal reaction involves the breakdown of both the oocyte’s plasma membrane and the sperm’s acrosomal membrane. The hyaluronidase and acrosin (another proteolytic enzyme) then digest a path through the zona pellucida towards the surface of the oocyte. Hundreds of acrosomes must undergo exocytosis to digest holes in the zona pellucida. This is one case that does not bear out the adage, “The early bird catches the worm.” A sperm that comes along later, after hundreds of sperm have undergone acrosomal reactions to expose the oocyte membrane, is in the best position to be the fertilizing sperm. Blocks to Polyspermy Previously while the sperm was binding to the sperm receptor [ZP3 glycoprotein] it induced further calcium influx into the sperm. This explains that when the spermatozoa membrane fuses with the oocyte membrane the levels of calcium within the oocyte are now elevated. This triggers 3 main events, 2 of which are blocks to polyspermy, Fast and Slow. Fast – The influx of calcium causes the oocyte cell membrane to depolarize. This prevents membrane fusion with additional spermatozoa. Slow – The second or slow block to polyspermy is known as the cortical reaction. Cortical granules lie just beneath the oocyte cell membrane, and with the cortical reaction they fuse with the membrane and release their contents into the zoa pellucida. This hardens the zona and impairs the ability of sperm to bind the zona. Polyspermy results in a hydatiform mole (molar pregnancy) Once a path has been cleared and a single sperm fuses with the oocyte’s membrane receptors, its nucleus is pulled into the oocyte cytoplasm. Each sperm carries a special two-part binding apparatus on its surface. The beta protein part acts first as it binds to a receptor on the oocyte membrane. This event engages the alpha protein part, causing it to insert into the membrane. This somehow causes the egg and sperm membranes to open and fuse together with such perfect contact that the contents of both cells are combined within a single membrane—all without spilling a drop. Interestingly, the region of the oocyte where the sperm enters determines the future right and left axes of the embryo’s body. Completion of Meiosis II and Fertilization After a sperm enters the oocyte it loses its tail and midpiece, and the centrosome in its midpiece elaborates microtubules which the sperm uses to find the oocyte nucleus. The sperm then locomotes its DNA-rich nucleus toward the oocyte, its nucleus swelling to about five times its normal size to form the male pronucleus on the way (see above). Meanwhile the secondary oocyte, stimulated into activity by the calcium surges, completes meiosis II, forming the ovum nucleus and the second polar body. This accomplished, the ovum nucleus swells, becoming the female pronucleus, and the two pronuclei approach each other. A mitotic spindle develops between them (see above), and the pronuclei membranes rupture, releasing their chromosomes into the immediate vicinity of the newly formed spindle. The true moment of fertilization occurs as the maternal and paternal chromosomes combine and produce the diploid zygote, or fertilized egg. Almost as soon as the male and female pronuclei come together, their chromosomes replicate. Then, the first mitotic division of the conceptus begins. The embryo now contains a diploid number of chromosomes and 2N quantity of DNA and is called a zygote (and 3 polar bodies) 2. Outline and describe the cell cycle and indicate regulatory signals which control the entry of cells into and out of the cycle. The cell-cycle is highly regulated, and CHECKPOINTS control transitions between cell-cycle stages. Check points are biochemical circuits that detect external or internal problems and send inhibitory signals to the cell-cycle system. The cell-cycle control system is based on two families of proteins: the CYCLIN-DEPENDENT KINASES (Cdk) and the CYCLINS. REVISION: The cycle is divided into two major periods: beginning with interphase: G1 phase, followed by S phase, then G2 phase, which then leads to mitosis: the process of nuclear division. Interphase G1 phase (G=gap): The interval between the completion of mitosis and the start of S phase. S phase (S=synthesis): The portion of interphase in which DNA replication occurs. G2 phase After the S phase and before mitosis, the DNA is checked prior to mitosis. Mitosis In early mitosis (i.e. prophase and prometaphase) the nuclear envelope breaks down, the contents of the nucleus condense into visible chromosomes, and the cell's microtubules reorganize to form the mitotic spindle. Then, the cell seems to pause at metaphase, in which the duplicated chromosomes are aligned on the mitotic spindle, poised for segregation. At this point, the cell "can decide" whether to stop cell division or not, however, past metaphase, there is no return and the process is taken till two daughter cells form. Anaphase marks the beginning of chromosome segregation, which will be followed by telophase and eventually, by cytokinesis, the separation of the two cells by division of the plasma membrane. This marks the end of the mitotic period, also known as the M phase. THERE ARE 4 MAJOR TYPES OF CHECKPOINTS Restriction Point The Restriction Point in the G1 phase is sensitive to the physiological state of the cell and to its interactions with the surrounding extracellular matrix. Cells that do not receive appropriate growth stimuli from their environment do not progress past this point in the G1 phase and may commit suicide by apoptosis. DNA Damage Checkpoints DNA damage checkpoints operate in G1, S and G2 phases of the cell-cycle. In general, these checkpoints block cell-cycle progression, but they can also trigger cell death by apoptosis. (In general, DNA damage checkpoints block cell-cycle progression by inhibiting the cyclindependent kinases by a variety of mechanisms). DNA Replication Checkpoint The DNA Replication checkpoint detects the presence of unreplicated or stalled DNA replication forks. This checkpoint shares some components with the DNA damage checkpoints but has the additional feature that it specifically stabilizes stalled replication forks so that they can be repaired. Spindle Assembly Checkpoint During Mitosis, the spindle assembly checkpoint (metaphase checkpoint) delays the onset of chromosome segregation until all chromosomes have attached properly to the mitotic spindle. HOW THE CHECKPOINTS FUNCTION (Sensors > Transducers > Effectors) The checkpoints in G1, S and G2 use common strategies to regulate cell-cycle progression. - DNA damage is detected by sensors - These activate transducers (these are often protein kinases) - The transducers act on effectors - These effectors ultimately block cell-cycle progression and may fulfil other functions CYCLIN-DEPENDENT KINASES Genetic Analysis of the cell-cycle identified a gene called cdc2+ (cell division cycle-2+) that is essential for cell-cycle progression during both G1 > S and G2 > M transitions. The product of this gene is the protein kinase; Cdk1. Cdk1 is the prototype for a family of protein kinases that is crucial for cell-cycle progression in all eukaryotes. Humans have more than 10 distinct protein kinases related to Cdk1, although only a few are related to cell-cycle control. To be active, these enzymes must each associate with a regulatory subunit called a cyclin. Thus they have been termed cyclin-dependent kinases (Cdks) Cdk1 – functions primarily in the regulation of the G2 > M transition Cdk2 – is involved in both G1 > S and G2 > M transitions Cdk4 + Cdk6 – are involved in passage of the restriction point Cdk7 – is important for activation of other Cdks CYCLINS The defining feature of Cyclin-dependent kinases (Cdks) is that they require binding of cyclins for catalytic activity. Cyclins are a diverse group of proteins. Cyclins owe their name to the fact that they undergo a cycle of synthesis and degradation with each division cycle. The cyclic assembly, activation, and disassembly of cyclin-Cdk complexes are the pivotal events that drive the cell cycle.