CHM 1411 Chapter 4.doc
advertisement
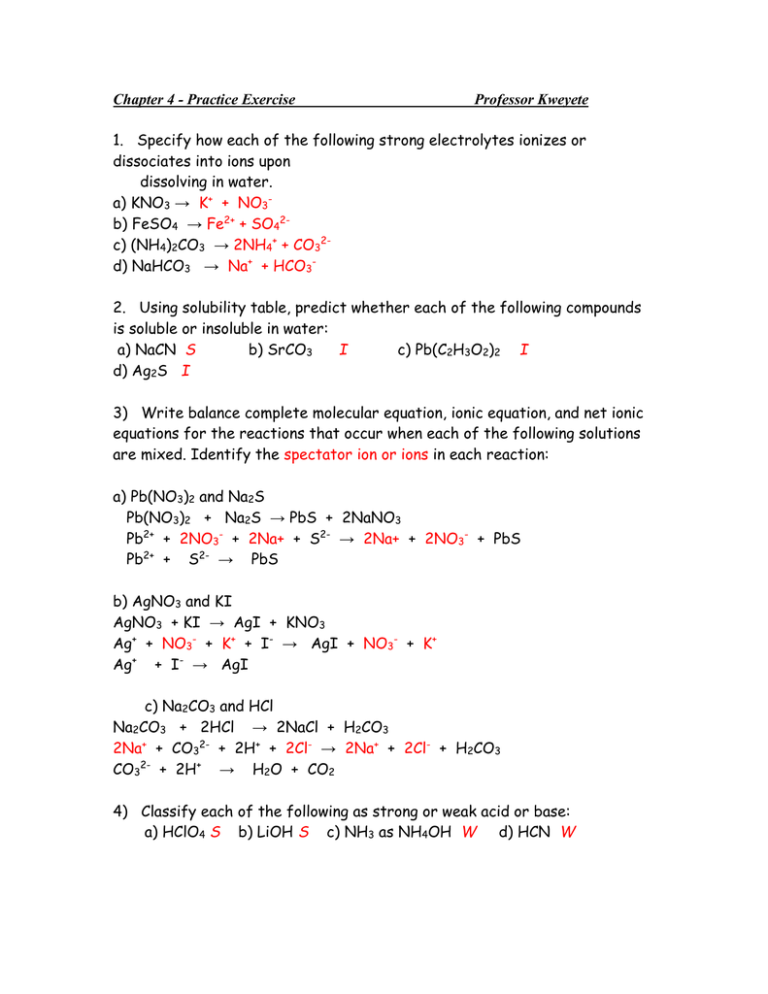
Chapter 4 - Practice Exercise Professor Kweyete 1. Specify how each of the following strong electrolytes ionizes or dissociates into ions upon dissolving in water. a) KNO3 → K+ + NO3b) FeSO4 → Fe2+ + SO42c) (NH4)2CO3 → 2NH4+ + CO32d) NaHCO3 → Na+ + HCO32. Using solubility table, predict whether each of the following compounds is soluble or insoluble in water: a) NaCN S b) SrCO3 I c) Pb(C2H3O2)2 I d) Ag2S I 3) Write balance complete molecular equation, ionic equation, and net ionic equations for the reactions that occur when each of the following solutions are mixed. Identify the spectator ion or ions in each reaction: a) Pb(NO3)2 and Na2S Pb(NO3)2 + Na2S → PbS + 2NaNO3 Pb2+ + 2NO3- + 2Na+ + S2- → 2Na+ + 2NO3- + PbS Pb2+ + S2- → PbS b) AgNO3 and KI AgNO3 + KI → AgI + KNO3 Ag+ + NO3- + K+ + I- → AgI + NO3- + K+ Ag+ + I- → AgI c) Na2CO3 and HCl Na2CO3 + 2HCl → 2NaCl + H2CO3 2Na+ + CO32- + 2H+ + 2Cl- → 2Na+ + 2Cl- + H2CO3 CO32- + 2H+ → H2O + CO2 4) Classify each of the following as strong or weak acid or base: a) HClO4 S b) LiOH S c) NH3 as NH4OH W d) HCN W 5) Determine the oxidation number for the indicated elements in each of the following substances: b) C in C2O42-2 = 4(-2) + 2C C = +3 d) P in H2PO4-1 = 4(-2) + 2(+1) + P P = +5 a) Br in HBrO2 0 = 2(-2) + 1(+) + Br Br = +3 c) C in COCl2 0 = -2 + 2(-1) + C C = +4 6) Which element is oxidized (Reducing agent), and which is reduced (Oxidizing agent) in the following reactions? a) Cl2 R + 2NaBr O 2NaCl + Br2 b) Zn O + Zn Cl2 Cl2 R c) Fe2O3 R + 3CO O 2Fe + 3CO2 7) Using activity series table, what is the outcome of each of the following reactions? If no reaction occurs, simply write NR. a) Ag b) Zn c) H2 d) Br2 f) Co + + + + + Pb(NO3)2 2AgNO3 CuO NaF 2HCl NR Zn(NO3 )2 + 2Ag H2O + Cu NR CoCl2 + H2 8) Calculate the following quantities: a) Molarity of a solution that contains 0.0225 mol Na2SO4 in 400 ml solution M = 0.0225 mol = 0.0563M 0.4L b) Moles of HNO3 in 45.0 ml of 2.50 M solution of nitric acid 2.50 mol│ 0.045L = 0.113 mole L │ c) Milliliters of 2.00 M Ca(OH)2 solution needed to supply 0.250 mol of Ca(OH)2 1L │0.25 mol = 0.125L = 125 ml 2 mol │ 9) Indicate the concentration of each ion present in the following solution: a) 0.25 M BaCl2 => 0.25M Ba+2 ,0.5M Clb) 0.25 M C2H5OH => same c) 0.20 M KCl + 0.25 M K2CO3 => 0.70M K+, 0.20M Cl-, 0.25M CO32d) 20 ml of 0.20 M HCl + 10.0 ml of 0.500 M HCl 20ml(0.2M) + 10ml(0.5M) = 0.3M H+ & 0.3M Cl20ml + 10ml 10) Calculate the following quantities: a) milliliters of 0.75 M HCl required to neutralized completely 25.0 ml of 0.15 M Ba(OH)2 1(0.75M)Va = 2(0.15M)(25 ml); Va = 10 ml b) if 30.0 ml of 0.75 M HCl solution is needed to neutralize a solution of Ca(OH)2, how many grams of Ca(OH)2 must be in the solution? 1(30ml)(0.75M) = 2MbVb; MbVb = 0.0113 mole; g = 0.0113(74g/mol) = 0.84g 11) How would you prepare 100.0 ml of 0.045 M Na2SO4 solution starting with pure solid? 0.045M(0.1L) = 0.0045mole; mol wt = 142g/mol 0.0045(142) = 0.64g So weigh out 0.64g of solid, place in a 100ml volumetric flask and add water to the 100ml mark to make the solution.






