Chemistry Essentials
advertisement
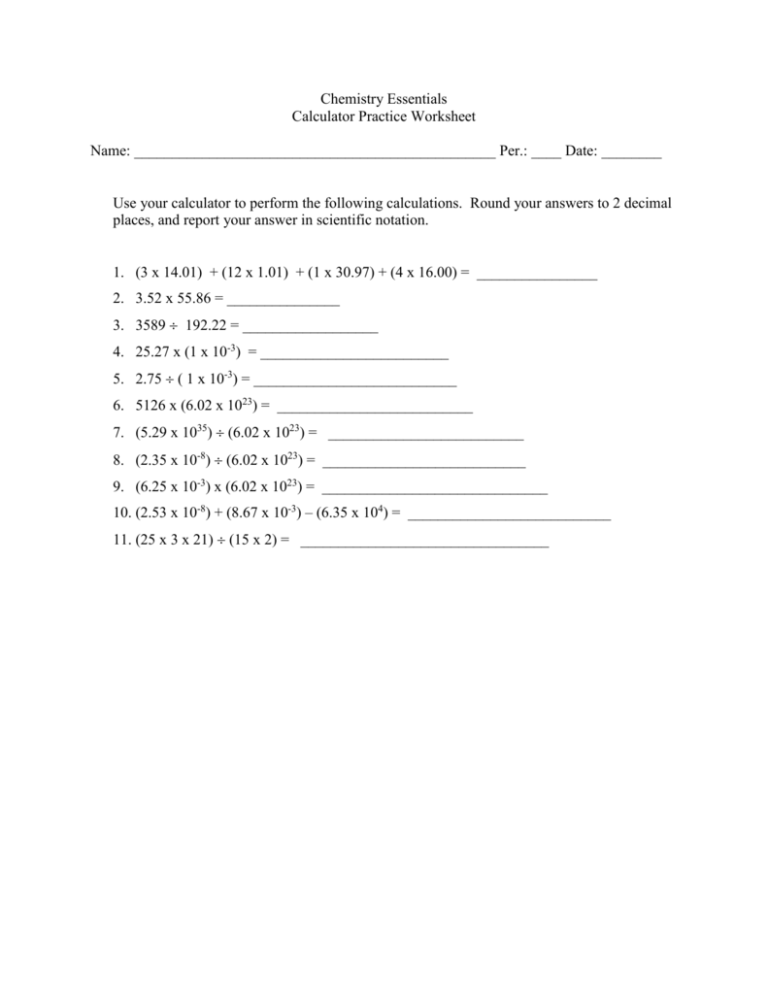
Chemistry Essentials Calculator Practice Worksheet Name: ________________________________________________ Per.: ____ Date: ________ Use your calculator to perform the following calculations. Round your answers to 2 decimal places, and report your answer in scientific notation. 1. (3 x 14.01) + (12 x 1.01) + (1 x 30.97) + (4 x 16.00) = ________________ 2. 3.52 x 55.86 = _______________ 3. 3589 192.22 = __________________ 4. 25.27 x (1 x 10-3) = _________________________ 5. 2.75 ( 1 x 10-3) = ___________________________ 6. 5126 x (6.02 x 1023) = __________________________ 7. (5.29 x 1035) (6.02 x 1023) = __________________________ 8. (2.35 x 10-8) (6.02 x 1023) = ___________________________ 9. (6.25 x 10-3) x (6.02 x 1023) = ______________________________ 10. (2.53 x 10-8) + (8.67 x 10-3) – (6.35 x 104) = ___________________________ 11. (25 x 3 x 21) (15 x 2) = _________________________________ Chemistry Essentials Measurement and Calculations Conversions Worksheet Name: _____________________________________________ Per.: _____ Date: ______ Make the following conversions. Show all your work (as demonstrated in class) on a separate piece of paper and attach it to the back of this paper. Report all answers in scientific notation. Round the coefficient to two decimal places 1. 3.4 liters to milliliters _____________________ 2. 876 millimeters to meters __________________ 3. 78,999 milligrams to grams ________________ 4. 0.9 centigrams to grams ___________________ 5. 112 meters to millimeters __________________ 6. 45 meters to centimeters___________________ 7. 11.7 grams to kilograms ___________________ 8. 0.0009 kiloliters to liters ___________________ 9. 44 centimeters to meters ___________________ 10. 277 kilograms to grams ____________________ 11. 16.7 inches to cm _____________________ 12. 25 yards to meters __________________ 13. 65 years to seconds __________________ 14. 84 miles to kilometers __________________ 15. 4.75 centimeters to inches _______________ 16. 48,987 minutes to days __________________ 17. 29.6 gallons to milliliters ___________________ 18. 6552 milliliters to quarts ____________________ 19. 4.56 1032 kilograms to milligrams ________________ 20. 9.58 x 1012 kilometers to millimeters _________________ 21. 5.2369 x 10-6 kilograms to micrograms _________________ 22. 6.97896 x 108 milliliters to megaliters ____________________ 23. 2.36951 x 1025 grams to kilograms ______________________ 24. 1.233 x 10-6 liters to milliliters _________________________ 25. 2.612 x 109 grams to milligrams_________________ Useful Information 1 inch = 2.54 cm 1 mile = 1.61 km 1 lb = 454 g 1 liter = 1.06 qt 1 gal = 3.78 liters 1 yard = 3.00 feet Chemistry Essentials Molar Mass Worksheet Name: ______________________________________________Per._____ Date: _______ Calculate the molar mass(grams/mole) for each of the following compounds. SHOW ALL YOUR WORK USING THE METHOD SHOWN IN CLASS. 1. KMnO4 2. CH4 3. P2O7 4. (NH4)2SO4 5. FeCl3 6. CuOH 7. Mg3(PO4)2 8. C6H12O6 9. Cr2(Cr2O7)3 10. Sn(C2H3O2)4 Chemistry Essentials Mole Worksheet Name: _____________________________________________ Per.: ____ Date: ________ Answer the following questions. SHOW ALL YOUR WORK USING THE METHOD TAUGHT IN CLASS. 1. How many ducks are there in 5.25 moles of ducks? 2. How many grains of sand are there in 3.25 x 10-5 moles of grains of sand? 3. How many stuffed animals are in 6.50 x 10-24 moles of stuffed animals? 4. How many cookies are there in 1.93 x 103 moles of cookies? 5. How many bacteria are there in 1.49 moles of bacteria? 6. How many moles of koalas are in 1.50 x 105 koalas? 7. How many moles of viruses are in 1.0 x 1031 viruses? 8. How many moles of rocks are in 2.50 x 103 rocks? ( That is 2500 rocks) 9. How many moles of students are in 1950 students? 10. How many moles of people are on the earth, if the population of the earth is 7.01 x 109 people? Chemistry Essentials Measurement and Calculations Rounding Decimals Worksheet Name: _____________________________________________ Per.: _____ Date: ______ Round to the nearest tenth (1 decimal place): 1) 2) 3) 4) 5) 6) 0.457 __________ 2.793 __________ 0.124 __________ 0.8156 _________ 3.456 __________ 0.317 __________ 7) 8) 9) 10) 11) 12) 0.672 __________ 4.321 __________ 0.543 __________ 0.93 __________ 12.57 __________ 0.781 __________ 19) 20) 21) 22) 23) 24) 0.672 __________ 4.321 __________ 0.543 __________ 0.93 __________ 12.57 __________ 0.781 __________ Round to the nearest hundredth (2 decimal places): 13) 14) 15) 16) 17) 18) 0.457 __________ 2.793 __________ 0.124 __________ 0.8156 _________ 3.456 __________ 0.317 __________ Round to nearest whole number (no decimal places): 25) 26) 27) 28) 29) 30) 31) 32) 1.4 __________ 8.34 _________ 2.8 __________ 9.91 _________ 3.458 __________ 7.123 __________ 4.67 __________ 12345.2567 __________ Chemistry Essentials Measurement and calculations Scientific Notation Worksheet Name: _____________________________________________ Per.: _____ Date: ______ Convert the following to scientific notation. Round the coefficient to two decimal places. 1) 45,700 _________________ 2) 0.009 ___________________ 3) 23 _____________________ 4) 0.9 _____________________ 5) 24,212,000 ______________ 6) 0.000665 ________________ 7) 21.9____________________ 8) 0.00332 _________________ 9) 321 ____________________ 10) 0.119___________________ 11) 1492 ___________________ 12) 0.2713__________________ 13) 314159__________________ 14) 6022 ___________________ 15) 0.12011_________________ Convert the following numbers in scientific notation to expanded (standard) form. 16) 3.825 x 103 ______________ 17) 6.3 x 104 ________________ 18) 2.3 x 10-2 _______________ 19) 4.44 x 10-6 ______________ 20) 7.121 x 109 ______________ 21) 1.2 x 10-1 _______________ 22) 1.8 x 102 ________________ 23) 8.1 x 10-4 _______________ 24) 6.7 x 105 ________________ 25) 3.4 x 107 ________________ Chemistry Essentials Stoichiometry Worksheet Moles → Moles Name: ___________________________________________________ Per.: _____ Date: ______ SHOW ALL YOUR WORK USING THE METHOD SHOWN IN CLASS. 1. The balanced chemical equation for the combustion of propane(C3H8) is C3H8(g) + 5O2(g)→ 3CO2(g) + 4H2O(g). a. How many moles of carbon dioxide will be formed from 2.5 moles of propane? b. How many moles of carbon dioxide will be formed from 2.5 moles of oxygen gas (O2)? c. How many moles of water will be formed from 5.9 moles of oxygen gas? d. How many moles of water will be formed from 5.9 moles of propane? e. How many moles of propane are needed to react completely with 3.75 moles of oxygen gas? f. How many moles of oxygen gas are needed to react completely with 3.75 moles of propane? g. How many moles of propane are needed to produce 1.85 moles of carbon dioxide? h. How many moles of oxygen gas are needed to produce 1.85 moles of carbon dioxide? i. How many moles of oxygen gas are needed to produce 6.83 moles of water? j. How many moles of propane are needed to produce 6.83 moles of water? Chemistry Essentials Stoichiometry Worksheet Mass → Mass Name: ___________________________________________________ Per.: _____ Date: ______ SHOW ALL YOUR WORK USING THE METHOD SHOWN IN CLASS. You only need to show molar mass calculations once. 2. The balanced chemical equation for the combustion of propane(C3H8) is C3H8(g) + 5O2(g)→ 3CO2(g) + 4H2O(g). a. How many grams of carbon dioxide will be formed from 2.5 grams of propane? b. How many grams of carbon dioxide will be formed from 2.5 grams of oxygen gas (O2)? c. How many grams of water will be formed from 5.9 grams of oxygen gas? d. How many grams of water will be formed from 5.9 grams of propane? e. How many grams of propane are needed to react completely with 3.75 grams of oxygen gas? f. How many grams of oxygen gas are needed to react completely with 3.75 grams of propane? g. How many grams of propane are needed to produce 1.85 grams of carbon dioxide? h. How many grams of oxygen gas are needed to produce 1.85 grams of carbon dioxide? i. How many grams of oxygen gas are needed to produce 6.83 grams of water? j. How many grams of propane are needed to produce 6.83 grams of water?




