Engineering Physics II
advertisement

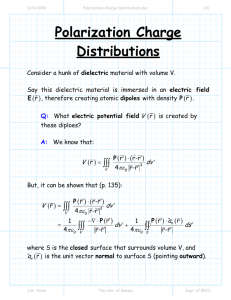
SRI RAMAKRISHNA INSTITUTE OF TECHNOLOGY COIMBATORE-10 (Approved by AICTE, New Delhi & Affiliated to Anna University) DEPARTMENT OF SCIENCE AND HUMANITIES Course Code &Title Class Regulation Course Prerequisite Programme Educational Objectives Course Outcome Program Outcomes Mapping PH 6251 & Engineering Physics- II First Year, B.E- Electronics and Communication Engineering-B Semester L P T C 3 0 0 3 02 Anna University, Chennai, R 2013 Fundamental concepts and knowledge about Engineering Physics - I. Knowledge of basic mathematical functions and formula Atomic Structure of Materials and its behavior PEO 1: Excel in professional career and/or higher education by acquiring knowledge in mathematical, scientific and engineering principles. PEO 2: Analyze real life problems, design electrical systems appropriate to its solutions that are technically sound, economically feasible and socially acceptable. PEO 3: Exhibit professionalism, ethical attitude, communication skills, team work in their profession and adapt to recent trends through continuous learning. of basic mathematical CO.1 ToKnowledge gain knowledge on the conductingfunctions materials.and formula Atomic structure of materials and its behavior. CO.2 To understand the concept of semiconducting materials and its types. CO.3 To learn the properties of magnetic and superconducting materials. CO.4 To acquaint with the basic nature of dielectric materials. CO.5 To impart knowledge on modern engineering materials. PO 1: an ability to apply knowledge of Mathematics, Science and Engineering. PO 2: an ability to design and conduct experiments as well as to analyze and interpret data. PO 3: an ability to design a system, component or process to meet desired needs within realistic constraints such as economic, environmental, social, political, ethical, health and safety, manufacturability and sustainability. PO 4: an ability to function on multidisciplinary teams. PO 5: an ability to identify, formulate and solve engineering problems. PO 6: an understanding of professional and ethical responsibility. PO 7: an ability to communicate effectively. PO 8: the broad education necessary to understand the impact of engineering solutions in a global, economic, environmental and societal context. PO 9: a recognition of the need for and an ability to engage in life-long learning. PO 10:an ability to use the techniques, skills and modern engineering tools necessary for engineering practice. PO1 PO2 PO3 PO4 PO5 PO6 PO7 PO8 PO9 PO10 CO1 CO2 CO3 CO4 CO5 References e-Learning Resources : Mode of Evaluation Faculty TEXT BOOKS: 1. Arumugam M., Materials Science. Anuradha publishers, 2010 2. Pillai S.O., Solid State Physics. New Age International(P) Ltd., publishers, 2009 REFERENCES: 1. Palanisamy P.K. Materials Science. SCITECH Publishers, 2011 2. Senthilkumar G. Engineering Physics II. VRB Publishers, 2011 3. Mani P. Engineering Physics II. Dhanam Publications, 2011 4. Marikani A. Engineering Physics. PHI Learning Pvt., India, 2009 http://libguides.wpi.edu/physics http://libraryguides.lynchburg.edu/eresourcesphysics http://eresources.rhul.ac.uk/kb/Lecture_Notes_in_Physics http://www.uic.edu/depts/lib/science/resources/index.shtml http://library.stanford.edu/guides/materials-science-and-engineering-resources 1.Internal Assessment (20) Internal Assessment Test 1 will be conducted for 50 Marks. (5*2=10 & 2*16=40) Internal Assessment Test 2 will be conducted for 50 Marks. (5*2=10 & 2*16=40) Internal Assessment Test 3 will be conducted for 50 Marks. (5*2=10 & 2*16=40) (All the three test marks will be considered for assessment out of 20)Tests will be conducted as per the schedule given by the university. 2. External Assessment (80) University will conduct end semester examination for 100 marks (10*2=20 & 5*16=80) Performance will be considered for assessment out of 80. Dr.S.Vijayakumar, Associate Professor, Faculty in Physics COURSE PLAN UNIT TOPICS TO BE COVERED REFERENCE CONDUCTING MATERIALS Introduction- Conductors – Classical free electron theory of metals T1:1.1 – 1.4 I II T1:1.4 –1.5/R2:1.7– 1.8 T1:1.14 – 1.17 T1:1.17 – 1.21 T1:1.23 – 1.24 T1:1.30 – 1.31 T1:1.31 – 1.33 T1:1.33 – 1.37 TOTAL SEMICONDUCTING MATERIALS Introduction – Intrinsic semiconductor T1:2.5 -2.7/R2: 2.4-2.8 Intrinsic semiconductor – carrier concentration derivation T12.8 –2.12/R2:2.8-13 Variation of Fermi level with temperature - Electrical conductivity T1:2.12- 2.13R2:2.13-2.16 Band gap determination of intrinsic semiconductor – Compound T1:2.13 – 2.14,& 2.5-2.6/ semiconductors – Direct and Indirect Band gap R2: 2.17:2.18 Extrinsic semiconductors – carrier concentration derivation in n-type T1:2.7 – 2.8 &2.14- 2.17 Electrical Conductivity of conducting materials Thermal Conductivity of conducting materials Wiedemann , Franz law,Lorentz number– Drawbacks of classical theory Introduction Quantum theory Quantum theory – Fermi distribution function Effect of temperature on Fermi function Density of energy states – carrier concentration in metals. Extrinsic semiconductors – carrier concentration derivation in p-type semiconductor variation of Fermi level with temperature and impurity concentration Hall effect –Determination of Hall coefficient – Applications T1:2.17– 2.18/ R2: 2.26-2.28 T1:2.19–2.20/R2: 2.28-2.30 T1:2.20 – 2.25 TOTAL HOURS REQUIRED 1 1 1 1 1 1 1 2 9 1 1 1 1 1 1 1 2 9 MAGNETIC AND SUPERCONDUCTING MATERIALS III Origin of magnetic moment – Bohr magneton Comparison of dia, para and ferro magnetism Domain theory - Hysteresis T1:3.2 -3.3 T1:3.4 -3.6, 3.8- 3.9 T1:3.12 -3.18 1 1 1 IV V Soft and hard magnetic materials - Anti – ferromagnetic materials – Ferrites and its applications T1:3.21 -3.22/ R2:3.20-3.24 1 Superconductivity : Properties – Type I and Type II super conductors BCS theory of superconductivity High Tc superconductors Applications of superconductors,SQUID,Cryotron, Magnetic levitation T1:3.26 -3.31 T1:3.25 -3.26 T1:4.10- 4.14 T1:3.33 – 3.35/R2: 4.14-.18 TOTAL 2 1 1 1 9 DIELECTRIC MATERIALS Electrical susceptibility – dielectric constant T1:4.1- 4.3 Electronic Polarization T1:4.4- 4.7 Ionic Polarization T1:4.7- 4.4.8 Orientational and Space charge polarization T1:4.8- 4.13 Frequency and temperature dependence of polarization T1:4.13- 4.15 Internal field (derivation) T1:4.18- 4.21 Clausius – Mosotti relation (derivation) T1:4.21- 4.23 Dielectric loss – Dielectric breakdown T1:4.23 – 4.26 Uses of dielectric materials (capacitor and T1:4.27 – 4.32 transformer) – ferro electricity and applications. TOTAL ADVANCED ENGINEERING MATERIALS Metallic glasses: Preparation, properties and applications T1:5.2 – 5.5/R2: 6.2 -6.7 T1:5.5 - 5.6/R2: 6.8-6.12 Shape memory alloys (SMA): Characteristics Properties of NiTi alloy, application, R3: 6.13-6.14/T1:5.9 -5.11 Nano materials: Preparation - Chemical vapour deposition T1:5.12- 5.13 Pulsed laser deposition - Applications T1:5.14- 5.16,5.19 -5.21 NLO Materials T1:5.22- 5.23 Birefringence – Optical Kerr effect T1:5.23 – 5.24/R3 : 8.2-8.5 Classification of biomaterials and its applications R3: 8.8-8.14/T1: 5.29 – 5.31 TOTAL TOTAL HOURS: COURSE INSTRUCTOR HOD PRINCIPAL 1 1 1 1 1 1 1 1 1 9 2 1 1 1 1 1 1 1 9 45