Gas Lab
advertisement

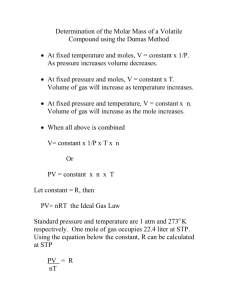
AP Chemistry Experiment – Molar Mass of a Volatile Liquid Objectives To observe the gaseous state of matter and determine the molar mass of a volatile liquid. Introduction To identify a new compound, a chemist must first identify its physical properties. These properties include: melting point, color, density, and elemental composition. In this experiment we will determine the molar mass of an organic compound. A number of methods can be used to determine the molar mass of a compound. In the case of compounds with large molecules such as proteins, drugs, and enzymes, an osmometer is used. In the case of smaller a mass spectrometer is used. For the case of volatile liquids as in the case of our lab the method developed by John Dumas (1800 – 1884) will be used. The unknown liquid in question is placed in a small flask of known volume. The flask is then placed into a beaker of boiling water. The liquid vaporizes and the number of moles is then calculated. The basic assumption of a ideal gas will be the basis of calculation. The pressure will be set to atmospheric. PV nRT Accuracy is very important to obtain good results in this experiment. The mass of the vapor can be determined by the following relation: mvapor m flask vapor _ m flask The molar mass of the compound can be determined by the following relation: m M compound vapor nvapor Gases and liquids with relatively large intermolecular forces and volumes do not follow the ideal gas laws or Kinetic Molecular Theory. Some compounds such as water deviate significantly from the ideal gas law. Under these conditions Van der Waal’s equation can be used in place of the ideal gas law. This equation accounts for intermolecular forces. n2 a ( P 2 )(V nb) nRT V The values for P, V, n, R, and T are the same as in the ideal gas law. The values for “a” and “b” represent constants accounting for intermolecular forces. These values are expressed in the following table for some liquids. 1 Van der Waal Constants for some Low Boiling Point Liquids Name Methanol Ethanol Acetone Propanol Hexane Cyclohexane Pentane Water a L2 atm mole 2 9.523 12.02 13.91 14.92 24.39 22.81 19.01 5.46 b L mole 0.06702 0.08407 0.0994 0.1019 0.1735 0.1424 0.1460 0.03051 Boiling Point Degrees C 65.0 78.5 56.5 82.4 69.0 80.7 36.0 100.0 Pre Lab Questions Answer the following questions on another sheet of paper. 1. Explain how the mass and vaporized liquid is determined by this experiment? 2. Define molar mass. 3. A .149 gram sample of un known vapor occupies a flask at 89 degrees C, 771 torrs, and a volume of 148 ml. Assume Ideal Gas Behavior. How many moles of the vapor are present? What is the molar mass of the vapor? 4. Explain the meaning of the values for “a” and “b”. Experimental Procedure 1. Determine the total volume inside the flask by filling it with water to the brim and then measuring the volume of water. Prepare a 125 ml flask by thoroughly cleaning it and drying it. Cover the end of the flask with a piece of Aluminum Foil. Hold the foil in place with a rubber band. 2. Measure the mass of the flask with rubber band and foil. 3. Uncover the flask and place 5 ml of unknown liquid inside the flask and place the foil over the end of the flask, secure the foil with the rubber band. 2 4. Using a 600 ml beaker add enough water to act as a heating bath so that when the flask is inserted, enough space remains to allow proper boiling of water. See instructor. 5. Puncture several pin size holes through the foil to allow the gas inside to always be at atmospheric pressure. 6. Gently heat the beaker to boiling. Be sure you have only a gentle boiling of the water. 7. Constantly monitor the temperature of the water. 8. When all of the unknown liquid appears to disappear from the flask, remove it from the beaker. Allow the flask to cool be placing it in ice. 9. Dry the outside of the flask carefully and measure the mass of the flask, foil, and unknown gas. 10. Perform this experiment 3 times to ensure accurate data. Data Sheet Mass of flask, rubber band, and foil ________ ________ ________ Temperature of boiling water ________ ________ ________ Mass of dry flask + foil assembly ________ ________ ________ Volume of flask ________ ________ ________ Atmospheric Pressure ________ ________ ________ Calculations Amount of vapor nvapor ________ ________ ________ Mass of vapor mvapor ________ ________ ________ Average molar mass ________________ Unknown liquid _________________________ 3 Standard deviation Show calculations for one trial + error analysis Show all calculations for all trials on another sheet of paper. Post Lab Questions Answer the following questions on another sheet of paper. 1. What would be a suitable hypothesis for this experiment? 2. What would be a suitable conclusion for this experiment? 3. Explain all your errors. 4. Can the assumption that the unknown vapor in the flask is an Ideal Gas be used? Why/Why not? 5. Can this technique be used to find the molar mass of all alcohols? Why/Why not? 4